Circular 676
Robert Flynn
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Author: Extension Agronomist, Department of Extension Plant Sciences, New Mexico State University. (Print friendly PDF)
Introduction
A soil test is an important management tool for developing an efficient soil fertility program, as well as monitoring a field for potential soil and water management problems. A soil test provides basic information on the nutrient-supplying capacity of the soil. However, a test is not reliable if the soil sample is taken incorrectly or improperly handled after collection. Please refer to NMSU Extension Guide A-114, Test Your Garden Soil (https://pubs.nmsu.edu/_a/A114.pdf), for information on how to properly sample soil. County Extension agents (https://aces.nmsu.edu/county/) can assist you with soil sample collection, submission, and interpretation of test results.
Because analytical techniques vary among laboratories, the values reported may vary from lab to lab. The numbers used by each lab have specific meanings for the lab and for the region in which the lab is located. The interpretations discussed here are for those methods reported in NMSU Extension Guide A-146, Appropriate Analyses for New Mexico Soils (https://pubs.nmsu.edu/_a/A146.pdf), as well as other tests that might be requested. Appropriate analyses are summarized in this publication, and the interpretation is presented for New Mexico soils.
Fertilizer and soil management recommendations shown on a soil test report are based on the soil test and information garnered from local, state, or regional nutrient application trials. Laboratories will usually send fertilizer recommendations, if needed, with the soil test results. As the client, you should do your best to submit your cropping history, previous yields, amount and type of fertilizer applied, depth of soil and depth to water table, water quality, and irrigation practices. Water quality is especially important for private well water. Additional comments on your sample submission form can include general appearance of the crop, problems that may have a bearing on the crop, and the depth to which the sample was taken. Fertilization requirements can vary with the overall crop management program. Complete and accurate information is essential to optimize crop yield for the lowest cost.

Individual Soil Tests
The following classifications are used for the suggested soil test results conducted by any given laboratory using the appropriate procedures. Except for pH, the classifications are categorized as deficient, low, moderate, sufficient, and excessive. For fertility factors (N, P, K, and micronutrients), very low and low classifications indicate a high probability for obtaining a fertilizer response, moderate classifications indicate a fertilizer response may or may not occur, and high and very high classifications indicate a fertilizer response is not likely to occur. It is also valid to consider soils that test very low to low to be deficient in that nutrient, and those that test high are considered sufficient for plant growth. Values in the very high range may also be toxic to certain plants depending on the nutrient and the plant being grown. Potential toxicity is usually identified in the soil test report.
pH. NMSU recommends the saturated paste method for determining soil pH. There is a difference in soil pH depending on what method is used. The difference between 1:1 and saturated paste is shown in Figure 1. Most crops will grow satisfactorily on soils with a pH ranging from 6.2 to 8.3. Crops susceptible to iron and zinc deficiencies may be affected at pH levels above 7.5. In many areas of New Mexico, soil pH is controlled by the presence of soil lime. It is likely that soil with as little as 3% calcium carbonate will have a pH greater than or equal to 7.5 (Figure 2). Soils with more than 3% calcium carbonate are considered to have a high buffering capacity, and it is difficult to change the pH of these soils. Table 1 describes soil pH.
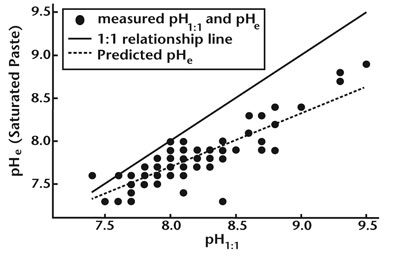
Figure 1. Relationship between saturated paste extract pH (pHe) and 1:1 soil:water extract pH (pH1:1) for the same soil (n = 97). The regression equation, pHe = 3.419 + 0.531(pH1:1) + 2.15 × 10-4(NH4OAc-Na) R2 = 0.76, can be used when pH1:1 is reported along with ammonium acetate (NH4OAc) extractable sodium to estimate pHe. The equation is valid only when soil saturation percentage (by weight) is between 24 and 51% and soil organic matter is between 0.6 and 3.4%. (Source: Robert Flynn, unpublished data from NMSU and private laboratory soil test results submitted to the author for interpretation.)
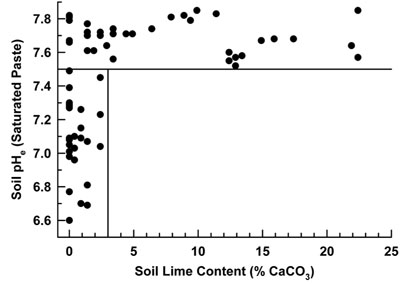
Figure 2. Saturated paste soil pH (pHe) versus soil lime content from New Mexico soils sampled to a depth of 12-inches submitted to the NMSU SWAT laboratory for analysis and interpretation by the author. Soil pHe at lime content above 3% will most likely be above 7.5.
|
Table 1. Soil pH Classification |
|
|
pH |
Classification |
|
>8.5 |
strongly alkaline |
|
7.9–8.5 |
moderately alkaline |
|
7.3–7.9 |
slightly alkaline |
|
6.7–7.3 |
neutral |
|
6.2–6.7 |
slightly acid |
|
5.6–6.2 |
moderately acid |
|
3.0–5.6 |
strongly acid |
Soil Lime (CaCO3). Many labs will report the presence of lime in the soil as low, medium, or high. Low corresponds to less than 1% lime, medium is 1 to 2%, and high is greater than 2%. Actual percentages are most useful if deciding whether or not to use elemental sulfur to either lower the soil pH or help as a reclamation tool under high sodium conditions. High levels of lime are also an underlying cause of chlorosis (yellowing between the leaf veins) in sensitive crops and ornamentals.
Salts, Electrical Conductivity (EC). The standard unit for conductivity is mmhos/cm or dS/m. Conductivity is best determined from a saturated paste extract (ECe). If ECe is reported in micromhos (µmhos) then divide by 1,000 when interpreting the numbers (Table 2). Soil with an ECe less than 1.5 mmhos/cm has few salinity problems. Table 2 classifies ECe into five levels. Problems may become evident in highly sensitive crops when the ECe is between 1 and 1.5, although problems are usually minor. Very few problems occur with ECe less than 1.0 mmhos/cm. When the ECe is above 1.5, problems usually are evident with sensitive plants. When the ECe is greater than 4, crops with moderate salt tolerance will usually show signs of reduced growth, foliage burn, or chlorosis. Leaching with low-EC water can decrease the salinity hazard if soil permeability is adequate (refer to NMSU Extension Guide W-102, Irrigation Water Analysis and Interpretation, https://pubs.nmsu.edu/_w/W102.pdf). The relative salt tolerance of selected crops (Table 3) and ornamental plants (Table 4) may be useful as a first approach to selecting plants based on soil salinity. Please refer to NMSU Extension Circular 656, An Introduction to Soil Salinity and Sodium Issues in New Mexico (https://pubs.nmsu.edu/_circulars/CR656.pdf), for more information on crop response to soil salinity and management.
|
Table 2. ECe Classification of Crop Tolerance (Mass, 1996) |
||
|
ECe |
† Estimated EC1:1 |
Classification |
|
mmhos/cm or dS/m |
||
|
<1.5 |
<0.84 |
very low – best for sensitive plants |
|
1.5–3 |
0.84–1.56 |
low – best for moderately sensitive plants |
|
3–4 |
1.56–2.03 |
moderate – best for moderately tolerant plants |
|
4–8 |
2.03–3.94 |
high – best for tolerant plants |
|
>8 |
>3.94 |
very high – best for very tolerant plants |
|
†It is best to request the saturated paste assessment for salinity, but if necessary ECe can be estimated from soil test reports that give EC1:1 and ammonium acetate extractable Na (ppm) by using the equation ECe = -0.0487 + 2.098(EC1:1) + 2.25 × 10-3(Na); n = 97, R2 = 0.86. Values for EC1:1 given above were calculated using Na = 97 ppm, which was the median of 97 samples from different regions of New Mexico (Source: unpublished data set from public and private samples submitted to NSMU SWAT lab for interpretation by R.P. Flynn). The equation is only an estimate and is valid for soils with saturation percentages (by weight) between 24 and 51% and EC1:1 between 0.5 and 8.14 mmhos/cm. |
||
|
Table 3. Relative Salt Tolerance of Selected Crops (Maas, 1996; National PLANTS Database [https://plants.usda.gov], accessed March 2014) |
|||
|
High Salt Tolerance |
Moderate Salt Tolerance |
Fair Salt Tolerance |
Low Salt Tolerance |
|
Field Crops |
|||
|
Barley (grain), cotton, guar, rye, sugar beet, triticale, wheat (durum and semi-dwarf) |
Cowpea, guar, oats (grain), rye (grain), safflower, sorghum (grain), soybean, wheat (grain) |
Broadbean, corn, flax, millet (foxtail), peanut, sunflower
|
Bean, sesame |
|
Forage Crops |
|||
|
Alkali sacaton, barley, bermudagrass, sprangletop (Malabar), ryegrass (Italian), trefoil (bird’s foot), wheatgrass (Siberian, slender, tall, and western), wildrye (Altai and beardless) |
Alfalfa (selected varieties), brome (mountain), clover (sweet), fescue (meadow and tall), Hardingrass, panicgrass (blue), rescuegrass, Rhodes grass, sorghum, sudangrass, wheatgrass (crested and intermediate), wildrye (Canada and Russian) |
Alfalfa, bentgrass, brome (smooth), buffelgrass, clover (alsike, berseem, ladino, red, strawberry, and white Dutch), corn (for silage), cowpea (for forage), foxtail (meadow), grama (blue), lovegrass, oatgrass (tall), oats (for forage), orchardgrass, rye (for forage), Timothy, vetch (winter) |
Rapeseed |
|
Vegetables |
|||
|
Asparagus |
Beet (red), zucchini |
Broccoli, Brussels sprouts, cabbage, cauliflower, celery, corn (sweet), cucumber, eggplant, kale, lettuce, pepper, potato, pumpkin, radish, spinach, squash (scallop), sweet potato, tomato, turnip |
Bean, carrot, okra, parsnip, pea
|
|
Fruit and Nut Crops |
|||
|
|
Pistachio, jujube |
Grape, muskmelon, watermelon |
Apple, apricot, blackberry, cherry, peach, pear, pecan, prune, plum, raspberry, strawberry |
|
Table 4. Tolerance of Selected Ornamental Plants to Soil Salinity (Maas, 1996; National PLANTS Database [https://plants.usda.gov], accessed March 2014) |
|
|
Tolerance and Range At Which Plants Are Affected |
Ornamental Plant |
|
Extremely sensitive |
Cotoneaster, rockspray (Cotoneaster horizontalis) |
|
Sensitive |
Dracaena, fragrant (Dracaena fragrans L.) Ker Gawl. |
|
Moderately tolerant |
Arborvitae (Thuja occidentalis L.) |
|
Tolerant |
Elaeagnus, thorny or Russian olive* (Elaeagnus angustifolia L.) |
|
Most tolerant |
Croceum iceplant, purple iceplant, rosea iceplant, white iceplant |
|
*Considered an invasive weed. |
|
Soil Organic Matter. Soil organic matter can be determined through either the loss on ignition or chemical oxidation method. If your soil contains lime or plant residues, the loss on ignition method may overestimate the amount of organic matter present. The chemical oxidation method is more accurate and more expensive. It has been used to estimate the percentage of organic matter that can supply nitrogen to a crop during a growing season. While the chemical oxidation method alone is not always a dependable measure of available nitrogen, it has been used with nitrate nitrogen levels to make nitrogen fertilizer recommendations for many crops. Generally, each percent of soil organic matter can be credited with providing 30 lb N/acre per growing season for crop use based on a 12-inch sampling depth.
Sandy soils also tend to have less organic matter compared to those with more clay. There is usually a strong relationship between soil organic matter, soil texture, and water-holding capacity (Hudson, 1994). However, soil organic matter has little effect on the overall water-holding capacity of clay soils. Table 5 has been adapted from Murphy et al. (2012) and classifies organic matter content according to textural classes. The ratings presented are suggested estimates for New Mexico.
|
Table 5. Suggested Interpretation of Soil Organic Matter Levels in New Mexico Soils According to Texture (Murphy et al., 2012) |
||||
|
Organic Matter Range |
Soil Textural Class |
|||
|
% |
Loamy Sand |
Sandy Loam |
Loam |
Silt Loam |
|
<0.5 |
Very low |
Very low |
Very low |
Very low |
|
0.5–1.0 |
Low |
Very low |
Very low |
Very low |
|
1.0–1.5 |
Medium |
Low |
Very low |
Very low |
|
1.5–2.0 |
High |
Medium |
Low |
Very low |
|
2.0–2.5 |
Very high |
High |
Medium |
Low |
|
2.5–3.0 |
Very high |
Very high |
Medium |
Medium |
|
3.0–4.0 |
Very high |
Very high |
High |
Medium |
|
4.0–5.0 |
Very high |
Very high |
Very high |
High |
|
>5.0 |
Very high |
Very high |
Very high |
Very high |
|
|
|
Sandy Clay, Silty Clay, |
|
|
|
<0.4 |
Very low |
Very low |
Low |
Very low |
|
0.4–0.8 |
Very low |
Very low |
Low |
Very low |
|
0.8–1.3 |
Very low |
Very low |
Medium |
Low |
|
1.3–1.8 |
Low |
Low |
Medium |
Medium |
|
1.8–2.3 |
Low |
Low |
High |
Medium |
|
2.3–2.8 |
Medium |
Medium |
High |
High |
|
2.8–3.3 |
Medium |
Medium |
Very high |
High |
|
>3.3 |
High |
High |
Very high |
High |
Texture. Not all laboratories evaluate soil texture as part of their normal fee structure. Many labs will estimate texture or perform a specific test to determine soil texture for an additional fee. Texture can be estimated at home or on the farm with the “feel” method by using the USDA–NRCS’s guide found in Gee and Bauder (1986) or https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/edu/?cid=nrcs142p2_054311.
General texture designations for the USDA Textural Classes are shown in Table 6. Coarse-textured soils (sands) have very low nutrient- and water-holding capacity. Fine-textured soils (clays) often have structural and infiltration problems.
|
Table 6. Soil Texture Designations |
|
|
USDA Textural Class |
General Texture |
|
Sand, loamy sand |
Coarse |
|
Sandy loam, fine sandy loam |
Moderately coarse |
|
Very fine sandy loam, loam, silt loam, silt |
Medium |
|
Sandy clay, silty clay, clay, silty clay loam, clay loam |
Fine |
Inorganic-Nitrogen. Soil can be tested for nitrate-nitrogen (NO3-N) and ammonium-nitrogen (NH4-N), which are both inorganic forms of nitrogen (N). It is important that the soil sample be air dried immediately after sampling to avoid changes in the inorganic-N concentration. Both of these ions are extractable by potassium chloride (KCl). Water extracts will not remove as much ammonium from the exchange sites in soils and may not represent the total inorganic-N in the soil. Nitrate-N, however, is the form most common in arable soils and is a measure of readily available nitrogen for plant use. Because NO3-N is highly soluble and has a negative charge, it is subject to leaching in all soils, but especially in coarse- to medium-textured soils. Ammonium-N does not accumulate in soil due to the effects of soil temperature and moisture that favor the conversion of NH4-N to NO3-N. Fertilizer recommendations for nitrogen are more accurate for certain crops if the subsoil (12–24 and 24–36 inches) is sampled in addition to the topsoil (8–12 inches, depending on crop). Applications of nitrogen fertilizer split over the course of the growing season help reduce the potential for leaching; split applications are particularly important for sandy or coarse-textured soils. Nitrogen fertilizer rates will vary greatly depending on what crop is being grown and how much residual nitrogen is available in the soil. Table 7 is a general classification for most crops.
|
Table 7. Soil Nitrate-N Interpretation |
|
|
Parts Per Million |
Classification |
|
<10 |
Deficient for most crops |
|
10–20 |
Low |
|
20–30 |
Moderate |
|
30–50 |
Sufficient |
|
>50 |
Excessive |
Bicarbonate Phosphorus (P). Bicarbonate phosphorus, also known as NaHCO3-P or Olsen-P, estimates plant-available P in alkaline (pH>7) soils (Table 8). Acidic (pH<7) soils should be analyzed using a different method extract such as the Bray. Soils in New Mexico are typically low in available phosphorus because it is quickly converted to insoluble calcium phosphate in the high-pH, high-calcium-content soils common in arid regions. Soils that have been amended with composts or manures often have sufficient levels of phosphorus for plant growth because compost and manure contain substantial quantities of organic P, which can be mineralized into inorganic P for crop use.
|
Table 8. Olsen-P Soil Test Interpretation |
|
|
Parts Per Million |
Classification |
|
<5 |
Deficient |
|
5–10 |
Low |
|
10–20 |
Moderate |
|
20–40 |
Sufficient |
|
>40 |
Excessive |
Cations
Extractable Potassium (K). In New Mexico soils, potassium is adequate for most crops and is not affected by high calcium carbonate content. Excessive K may be found in saline soils, but proper leaching and crop rotation can effectively manage both salts and K. The most common method to measure K availability in soil is the sum of the exchangeable and water-soluble K extracted by ammonium acetate and water solution. The water extraction gives the water-soluble K, while the ammonium acetate extraction gives both water-soluble plus exchangeable K. Table 9 shows ammonium acetate-extractable K as they are related to the classification scheme. Potassium fertilizer responses may sometimes be observed on sandy soils with low cation-exchange capacities and in crops that remove large quantities of K over the course of several years (perennials such as alfalfa and pasture grasses). Excessive soil K levels have been linked to elevated levels of K in grass forages, which can be detrimental to animal health. Low soil magnesium levels may also give rise to an imbalance of K relative to Ca and Mg for grass forages.
|
Table 9. Soil Test K Classification as Related to Two |
||
|
Parts Per Million K in Extract |
Classification |
|
|
Ammonium Acetate |
Water |
|
|
<150 |
<10 |
Low |
|
150–250 |
11–30 |
Moderate |
|
250–800 |
30–80 |
Sufficient |
|
>800 |
>80 |
Excessive |
Calcium (Ca) and Magnesium (Mg). Calcium deficiencies are rare, but have been known to occur in sandy soils for some New Mexico crops. In non-saline soils, there are limited amounts of soluble Ca and Mg. Calcium deficiency can also result from soils with a Ca/Mg ratio less than 0.5 (Rhoades, 2012). Most of the Ca and Mg in soil are exchangeable so that the lab results reflect both soluble and exchangeable. In soils that have been irrigated or where salts have accumulated, it is best to determine Ca and Mg from a saturated paste extract. This method is most reflective of soil solution Ca and Mg that is available to plants (Hartz et al., 2007). Furthermore, Hartz et al. (2007) found no significant correlation between soil solution Ca and ammonium-acetate-exchangeable Ca. Tables 10 and 11 classify Ca and Mg concentration, respectively, extracted from a saturated paste extract.
|
Table 10. Saturation Extract Soil Ca Interpretation |
|
|
Saturation Extract Ca (ppm) |
Interpretation |
|
<40 |
Less than sufficient |
|
40–60 |
Usually sufficient |
|
>60 |
More than sufficient |
|
Table 11. Saturation Extract Soil Mg Interpretation |
|
|
Saturation Extract Mg (ppm) |
Interpretation |
|
<8 |
Low |
|
8–12 |
Sufficient |
Sodium Adsorption Ratio (SAR) and Exchangeable Sodium Percentage (ESP). The saturated paste extract should also be analyzed for sodium, calcium, and magnesium in order to calculate SAR. NMSU Extension Circular 656, An Introduction to Soil Salinity and Sodium Issues in New Mexico (https://pubs.nmsu.edu/_circulars/CR656.pdf), explains this in greater detail, but suffice it to say that the higher the SAR, the more likely water will not infiltrate into the soil. However, the problem is also dependent on irrigation water salinity (Table 12).
A soil with too much sodium relative to calcium and magnesium is prone to develop problems with water infiltration. It is imperative that the saturated paste extract be used to determine whether or not the soil will have problems with water infiltration. Ammonium-acetate-extractable Ca, Mg, and Na failed to identify a sodium-affected soil 40% of the time (n = 100) compared to when the saturated paste was used. Additionally, the amount of amendment needed to correct sodium-affected soils was insufficient to meet reclamation requirements as determined by comparing calculated values from a saturated paste extract versus ammonium acetate. The general interpretation of SAR is given in Table 13.
ESP is the percentage of the soil cation exchange capacity occupied by sodium, and can be estimated from the SAR of the irrigation water where ESP = 1.475*(SARw)/(1 + 0.0147*(SARw)). As the percentage increases from 6 to 15%, there is an increase in the potential for the soil to experience poor water infiltration (Table 14).
High sodium concentration in the crop root zone can also cause poor plant growth for several crops. Table 15 gives some examples of sodium concentration tolerance for selected crops. Soils with a pHe of 8.5 or higher may also have high sodium content with a commensurate high sodium adsorption ratio (SAR).
|
Table 12. Effects of Soil SAR on Water Infiltration |
||
|
|
Potential water infiltration problem |
|
|
SAR of soil |
Unlikely if ECw is |
Likely if ECw is |
|
mmhos/cm or dS/m |
||
|
0–3 |
>0.7 |
<0.3 |
|
3.1–12 |
>2.0 |
<0.5 |
|
12.1–20 |
>3.0 |
<1.0 |
|
20.1–40 |
>5.0 |
<2.0 |
|
Table 13. Interpretation of SAR Determined from Saturated-Paste-Extractable Ca, Mg, and Na |
|
|
SAR |
Interpretation |
|
<6 |
Good; no amendments needed |
|
6–12 |
Poor; amendment recommendations usually given |
|
>12 |
Sodium-affected soil; reclamation required |
|
Table 14. Exchangeable Sodium Percentage (ESP) |
|
|
ESP |
Rating |
|
<6% |
Acceptable |
|
6–15% |
Elevated |
|
>15% |
Excessive |
|
Table 15. Tolerance of Various Crops to Soil Exchangeable Sodium Percentage (ESP)* in the Root Zone (Pearson, 1960) |
|
|
|
Growth response under field conditions |
|
Extremely sensitive |
Sodium toxicity symptoms |
|
Sensitive |
Stunted growth at these ESP values even though the physical condition of the soil may be good
|
|
Moderately tolerant |
Stunted growth due to both nutritional factors and adverse soil conditions |
|
Tolerant (ESP = 40–60; SAR = 46–100) |
Stunted growth usually due to adverse physical condition of soil |
|
Most tolerant |
Stunted growth usually due to adverse physical condition of soil |
|
*A comparable SAR was calculated for the given ESP should ESP not be reported in soil test results. |
|
Sulfur (S). Sulfur is generally not deficient in New Mexico. Most crops require between 20–30 lb S per acre. Plant-available S is released from organic matter and can also come from rainwater and irrigation water. If a soil test shows less than 8 ppm sulfate-S (SO4-S), a trial application of 10–20 lb S/acre could be done. Some laboratories will recommend S application at this soil level depending on crop and yield goals. However, testing for extractable SO4-S or other S forms has a poor relationship with S sufficiency for crops, and is not reliable in soils of many regions for predicting yield response to applied S. This is due to the presence of other sources of sulfate such as organic matter and irrigation water. Despite this issue, soil SO4-S interpretations are offered in Table 16. Knowing what other sources of sulfur there are, such as irrigation water and soil organic matter, may help you further understand the need for sulfur. NMSU Extension Circular 650, Sulfur and New Mexico Agriculture (https://pubs.nmsu.edu/_circulars/CR-650.pdf), contains more information
regarding sulfur in New Mexico.
|
Table 16. Sulfate-Sulfur Interpretation (adapted from Hornek et al., 2011) |
|
|
Parts Per Million |
Interpretation |
|
<2 |
Deficient |
|
2–20 |
Moderate |
|
>20 |
Sufficient |
DTPA-Extractable Iron. DTPA is a weak organic acid that can chelate iron and other metals, and represents a good estimate of plant-available metals in soils. Iron deficiency is often a problem with sensitive crops grown in soils with pH values over 7.5. Although the critical level of iron in soils is 4.5 ppm, iron-sensitive crops can often be grown satisfactorily down to levels of 2.5 ppm if rooting is not restricted by caliche (a calcic horizon) or gypsum, and care is taken to not over-irrigate (saturated soil conditions). Some plant species or varieties are more susceptible to iron deficiency than others, especially in the presence of soil lime. Examples of some plants that are prone to showing iron deficiency in high-pH soils are found in Table 17. Iron content classifications can be found in Table 18. Iron applications to high-pH soils are inefficient unless a chelated form is used (e.g., DTPA or EDDHA, sold as “iron chelate”). Foliar applications are generally recommended to correct deficiencies, but soil applications of Fe-EDDHA have been successful as well.
|
Table 17. Plant Sensitivity to Iron Deficiency in the Presence of Soil Lime |
|
|
Tolerant |
Alfalfa, annual fescue, Apache plume, big bluestem, desert wheatgrass, honey mesquite, tall wheatgrass, ocotillo, Osage orange, sainfoin, sweet clover, Utah serviceberry |
|
Moderately Tolerant |
Apple, bermudagrass, corn, desert willow, summer grape, strawberry clover, white clover, red clover, common sotol, common sunflower, wheat, crested wheatgrass, slender wheatgrass, mountain brome, western chokecherry |
|
Moderately Sensitive |
Beans, oats, soybean, dallisgrass, garden vetch, grain sorghum, pearl millet, smooth brome, sorghum, sudangrass |
|
Sensitive |
Cowpea, roses, crimson clover, rapeseed |
|
Table 18. DTPA-Extractable Soil Iron Content Interpretation (Lindsay and Norvell, 1978) |
|
|
Parts Per Million |
Classification |
|
<2.5 |
Low |
|
2.5–4.5 |
Moderate |
|
4.6–10 |
Sufficient |
|
>10 |
Excessive |
DTPA-Extractable Zinc. Zinc deficiency is an important problem in some crops, particularly corn, grain sorghum, and pecans. It is especially a problem in soils with pH values over 7.5 or soils that have a long history of heavy P fertilization that test over 200 ppm P. Some crop varieties may be more sensitive to zinc deficiency than other varieties. Suggested soil test interpretation for zinc is given in Table 19.
|
Table 19. DTPA-Extractable Soil Zinc Interpretation (Lindsay and Norvell, 1978) |
|
|
Parts Per Million |
Classification |
|
<0.5 |
Low |
|
0.5–0.75 |
Moderate |
|
0.76–1.00 |
Sufficient |
|
>10 |
Possible Toxicity |
DTPA-Extractable Copper. Copper deficiencies have not been verified in New Mexico. Factors contributing to copper deficiencies include high organic matter, sandy texture, and high pH. However, copper toxicities have been identified in some fields in New Mexico. These are often associated with fields that have received continuous applications of manure and/or effluent water from dairies. Copper toxicities have also been known to occur in summer grass crops like corn or sorghum if soil test levels exceed 12 ppm. Other plants may be more or less sensitive to copper. Table 20 classifies DTPA-extractable copper in terms of its sufficiency for plant growth.
|
Table 20. DTPA-Extractable Soil Copper Interpretation (Lindsay and Norvell, 1978) |
|
|
Parts Per Million |
Classification |
|
<0.2 |
Low |
|
0.2–0.3 |
Moderate |
|
0.31–0.6 |
Sufficient |
|
>12 |
Excessive, possible toxicity |
DTPA-Extractable Manganese. Manganese deficiencies have not been verified in New Mexico. They usually occur under conditions similar to those in which iron and zinc deficiencies occur. Manganese levels in the soil can also vary with soil moisture content. Refer to Table 21 to assess soil sufficiency for manganese.
|
Table 21. DTPA-Extractable Manganese Interpretation (Lindsay and Norvell, 1978) |
|
|
Parts Per Million |
Classification |
|
<1.0 |
Low |
|
1.0–2.5 |
Moderate |
|
2.6–4.0 |
Sufficient |
|
>4.0 |
Excessive, possible toxicity |
Hot-Water-Soluble Boron. Boron is an important micronutrient for plants and can be deficient in certain crops like alfalfa and peanuts. Nable et al. (1997) note that extraction methods that evaluate plant-available B at one point in time will be different from methods that evaluate the capacity of a soil to supply B. Chen et al. (2012) along with Maas (1987) have provided a summary of B tolerance based on plant response to B in soil solution, which is summarized in Table 22. Although there are various methods available to determine the levels of B in soils, it seems that soil analysis can provide little more than a general risk assessment for B toxicity. It is very difficult to utilize soil analysis to precisely predict the growth of plants on high-B soils (Nable et al., 1997). However, Bingham (1982) presented hot-water-soluble boron as a method to evaluate B deficiency. Boron toxicity level in soils is assessed by using a saturation extract with water (Richards, 1954). Levels in a saturated paste extract should not exceed 1.0 ppm, but some plants may be sensitive to levels below 0.5 ppm (Bingham, 1982). Hot-water-soluble boron is a common method employed by many soil testing labs and generally follows the classification in Table 23. However, certain crops grow quite well with hot-water-soluble boron levels less than 0.1 ppm, while others suffer from deficiency if levels drop below 1.0 ppm.
New Mexico soils vary in their B levels. Deficiencies have been observed in the Pecos Valley for some crops like pistachio. It is easy to apply too much B to correct a deficiency and induce toxicity. Follow label directions when applying B products to correct deficiency. Generally, soil levels that are less than 0.7 ppm from a saturated paste extract are safe for most plants.
|
Table 22. Relative Tolerance of Selected Plants to Boron Determined from Saturated Paste Extract (Chen et al., 2012) |
|
|
Relative Boron Tolerance |
Plants |
|
Very sensitive (0.05–0.75 ppm) |
Apricot (Prunus armeniaca L.), sweet cherry (Prunus avium L.), cowpea (Vigna unguiculata L.), grape (Vitis vinifera L.), pecan (Carya illinoinensis [Wangenh.] K. Koch), plum (Prunus domestica L.), onion (Allium cepa L.) |
|
Sensitive (0.75–1.0 ppm) |
Kidney bean (Phaseolus vulgaris L.), mung bean (Vigna radiata [L.] R. Wilczek), lima bean (Phaseolus lunatus L.), garlic (Allium sativum L.), peanut (Arachis hypogaea L.), strawberry (Fragaria spp. L.), wheat (Triticum aestivum L.) |
|
Moderately sensitive (1.0–2.0 ppm) |
Carrot (Daucus carota L.), cucumber (Cucumis sativus L.), pepper (Capsicum annuum L.), pea (Pisum sativum L.) |
|
Moderately tolerant (2.0–4.0 ppm) |
Barley (Hordeum vulgare L.), cabbage (Brassica oleracea L., Capitata group), cauliflower (Brassica oleracea L., Botrytis group), corn (Zea mays L.), zucchini squash (Cucurbita pepo var. melopepo L.), turnip (Brassica rapa L., Rapifera group) |
|
Tolerant (4.0–6.0 ppm) |
Alfalfa (Medicago sativa L.), purple vetch (Vicia benghalensis L.), red beet (Beta vulgaris L.), tomato (Solanum lycopersicum) |
|
Very tolerant (6.0–15.0 ppm) |
Asparagus (Asparagus officinalis L.), cotton (10 ppm) (Gossypium hirsutum L.), sorghum (6 ppm) (Sorghum bicolor L. [Moench]) |
|
Table 23. Hot-Water-Soluble Boron Classification (Grieve et al., 2012) |
|
|
Parts Per Million |
Classification |
|
<0.10 |
Deficient |
|
0.10–0.25 |
Low |
|
0.26–0.50 |
Marginal |
|
0.51–2.0 |
Sufficient |
|
2.1–6.0 |
High |
Chloride ion (Cl-). Chloride is an essential nutrient for plants. Most non-woody plants are not sensitive to chloride even at high concentrations, with the exception of certain soybean cultivars (Parker et al., 1983) that are not typically grown in New Mexico. Many woody species, however, are sensitive to chloride, but response to chloride varies among varieties and rootstocks within species. Chloride toxicity problems can be avoided by selecting rootstocks that exclude chloride from the scions. The interpretation of soil test chloride values should be based on a saturated paste extract. Table 24 presents the relative tolerance of selected plants to chloride.
|
Table 24. Relative Tolerance of Selected Plants to Chloride Determined from a Saturated Paste Extract (Maas and Grattan, 1999) |
||
|
Relative tolerance |
Maximum chloride concentration (ppm) before plant exhibits reduced growth |
Plants (Common name, scientific name) |
|
Very sensitive |
355 |
Kidney bean (Phaseolus vulgaris L.), carrot (Daucus carota L.), lettuce (Lactuca spp. L.), onion (Allium cepa L.), radish (Raphanus spp. L.), strawberry (Fragaria spp. L.), turnip (Brassica rapa L., Rapifera group) |
|
Sensitive |
532 |
Cabbage (Brassica oleracea L., Capitata group), corn (Zea mays L.), orchardgrass (Dactylis glomerata L.), pepper (Capsicum annuum L.), potato (Solanum tuberosum L.), red clover (Trifolium pratense L.), strawberry clover (Trifolium fragiferum L.), sweet potato (Ipomoea batatas [L.] Lam.) |
|
709 |
Alfalfa (Medicago sativa L.), sesbania (Sesbania herbacea (Mill.) McVaugh.), spinach (Spinacia spp. L.) |
|
|
Moderately sensitive |
886 |
Cucumber (Cucumis sativus L.), tomato (Solanum lycopersicum L.), broccoli (Brassica oleracea L. var. botrytis) |
|
1064 |
Beardless wildrye (Leymus triticoides [Buckley] Pilg.), scallop squash (Cucurbita pepo L.), sudangrass (Sorghum bicolor [L.] Moench ssp. drummondii [Nees ex Steud.] de Wet & Harlan) |
|
|
Moderately tolerant |
1241 |
Crested wheatgrass (Agropyron cristatum [L.] Gaertn.) |
|
1418 |
Tall fescue (Schedonorus arundinaceus [Schreb.] Dumort., nom. cons.), red beet (Beta vulgaris L.) |
|
|
1595 |
Zucchini squash (Cucurbita pepo var. melopepo, L.) |
|
|
1773 |
Cowpea (Vigna unguiculata [L.] Walp.), narrow-leaf birdsfoot trefoil (Lotus glaber Mill.) |
|
|
1950 |
Perennial ryegrass (Lolium perenne L.) |
|
|
Tolerant |
2127 |
Barley for forage (Hordeum vulgare L.), wheat (Triticum aestivum L.) |
|
2482 |
Sorghum (Sorghum bicolor [L.] Moench), bermudagrass (Cynodon dactylon [L.] Pers.) |
|
|
2659 |
Crested wheatgrass (Agropyron cristatum [L.] Gaertn.), cotton (Gossypium hirsutum L.), tall wheatgrass (Thinopyrum ponticum [Podp.] Z.-W. Liu & R.-C. Wang) |
|
|
2836 |
Barley for grain (Hordeum vulgare L.), grape (Vitis spp.) on Salt Creek 1613-3 or Dog Ridge rootstock |
|
Estimating Pounds Per Acre From PPM
Soil test results can be converted from parts per million (ppm) to pounds per acre by multiplying ppm by a conversion factor based on the depth to which the soil was sampled. Because a slice of soil that is 1 acre in area and 3 inches deep weighs approximately 1 million pounds, the conversion factors in Table 25 can be used.
|
Table 25. Multiplication Factors to Convert PPM to Pounds Per Acre for a Mineral Soil |
|||||||||||||
|
|
General approxi- |
|
|
|
|
|
|
Sandy clay loam |
|
|
|
Silty clay loam |
|
|
(inches) |
Multiply ppm by |
||||||||||||
|
3 |
1 |
0.92 |
0.95 |
0.98 |
0.98 |
0.97 |
1.01 |
1.02 |
0.99 |
0.93 |
0.84 |
0.88 |
0.93 |
|
6 |
2 |
1.84 |
1.90 |
1.95 |
1.95 |
1.93 |
2.02 |
2.05 |
1.98 |
1.86 |
1.68 |
1.76 |
1.86 |
|
7 |
2.33 |
2.15 |
2.22 |
2.28 |
2.28 |
2.26 |
2.35 |
2.39 |
2.31 |
2.18 |
1.97 |
2.06 |
2.18 |
|
8 |
2.66 |
2.46 |
2.54 |
2.60 |
2.61 |
2.58 |
2.69 |
2.73 |
2.64 |
2.49 |
2.25 |
2.35 |
2.49 |
|
9 |
3 |
2.77 |
2.85 |
2.93 |
2.93 |
2.90 |
3.02 |
3.07 |
2.97 |
2.80 |
2.53 |
2.65 |
2.80 |
|
10 |
3.33 |
3.07 |
3.17 |
3.25 |
3.26 |
3.22 |
3.39 |
3.41 |
3.30 |
3.11 |
2.81 |
2.94 |
3.11 |
|
12 |
4 |
3.69 |
3.80 |
3.90 |
3.91 |
3.87 |
4.03 |
4.09 |
3.96 |
3.73 |
3.37 |
3.35 |
3.73 |
|
Average bulk density (lb/ft3)† |
|
84.71 |
87.39 |
89.70 |
89.78 |
88.88 |
92.61 |
94.02 |
91.01 |
85.66 |
77.40 |
81.06 |
85.67 |
|
† Saxton and Rawls (2006) |
|||||||||||||
Fertility Considerations
A good soil sample and an accurate soil test interpretation are not the only considerations for optimal yields and profit in crop production. Even after applying recommended and appropriate amounts of fertilizer based on a soil test, other factors can override the effects of fertilizer by limiting the yield potential of a crop, including 1) soil texture; 2) control of weeds, insects, and diseases; 3) irrigation water quantity and quality; and 4) irrigation water management. Of these factors, the soil type and irrigation water quality are difficult for the grower to control. However, a good farmer can implement effective pest control and water management. Favorable fertilizer response is usually related to how well a crop is managed.
Summary
Table 26 provides a general summary of nutrient interpretations and classifications, toxicity levels, and optimal pH for specific soil nutrients.
|
Table 26. Soil Nutrient Interpretation, Toxicity Levels, and Optimal pH for Specific Nutrients |
|||||
|
|
|
|
|
|
|
|
ppm |
|||||
|
Nitrogen (N)† |
<10 |
10–30 |
30–50 |
>50 |
6.5–8 |
|
Phosphorus (P) |
<10 |
10–20 |
20–40 |
>100 |
6.5–8 |
|
Potassium (K) |
<150 |
150–250 |
>250 |
>800 |
6.5–8 |
|
Sulfur (S) |
<2 |
2–20 |
>20 |
N/A |
6.5–8 |
|
Boron (B) |
<0.25 |
0.25–0.50 |
0.51–2.0 |
>6 |
5–7 |
|
Chloride (Cl-) |
variable |
5–7 |
|||
|
Copper (Cu) |
<0.2 |
0.2–0.3 |
0.3–0.6 |
>12 |
5–7 |
|
Iron (Fe) |
<2.5 |
2.5–4.5 |
4.6–10 |
N/A |
5–7 |
|
Manganese (Mn) |
<1.0 |
1.0–2.5 |
2.6–4 |
>4 |
5–7 |
|
Zinc (Zn) |
<0.5 |
0.5–0.75 |
0.76–1.0 |
>10 |
5–7 |
|
† Depends on crop. Legumes, for example, produce their own nitrogen and often do not need additional input. Other problems can develop from high levels of nitrate-N, including leaching to groundwater under certain conditions. |
|||||
References
Ayers, R.S., and D.W. Westcot. 1985. Water quality for agriculture [FAO Irrigation and Drainage Paper 29, Rev. 1]. Rome: Food and Agriculture Organization of the United Nations.
Bingham, F. 1982. Boron. In A.L. Page et al. (Eds.), Methods of soil analysis, part 2, 2nd ed. (pp. 431–447). Madison, WI: American Society of Agronomy.
Chen, W.P., A.C. Chang, and A.L. Page. 2012. Deficiencies and toxicities of trace elements. In W.W. Wallender and K.K. Tanji (Eds.), Agricultural salinity assessment and management, 2nd ed. Reston, VA: American Society of Civil Engineers.
Flynn, R.P., and A. Ulery. 2011. An introduction to soil salinity and sodium issues in New Mexico [Circular 656]. Las Cruces: New Mexico State University Cooperative Extension Service.
Flynn, R.P., A. Ulery, and W.L. Lindemann. 2010. Sulfur and New Mexico agriculture [Circular 650]. Las Cruces: New Mexico State University Cooperative Extension Service.
Gavlak, R.G., D.A. Horneck, and R.O. Miller. 1994. Plant, soil and water reference methods for the Western Region [WREP 125]. Fairbanks: University of Alaska.
Gee, G.W., and J.W. Bauder. 1986. Particle-size analysis. In A. Klute (Ed.), Methods of soil analysis, part 1, 2nd ed. (pp. 383–409). Madison, WI: American Society of Agronomy.
Grieve, C.M., S.R. Grattan, and E.V. Maas. 2012. Plant salt tolerance. In W.W. Wallender and K.K. Tanji (Eds.), Agricultural salinity assessment and management, 2nd ed. Reston, VA: American Society of
Civil Engineers.
Hartz, K.H., P.R. Johnstone, R.F. Smith, and M.D. Kahn. 2007. Soil calcium status unrelated to tipburn of romaine lettuce. HortScience, 42, 1681–1684.
Hornek, D.A., D.M. Sullivan, J.S. Owen, and J.M. Hart. 2011. Soil test interpretation guide [EC1478]. Corvallis: Oregon State University Extension Service. Available at https://extension.oregonstate.edu/sorec/sites/default/files/soil_test_interpretation_ec1478.pdf
Hudson, B.D. 1994. Soil organic matter and available water capacity. Journal of Soil and Water Conservation, 49, 189–194.
Lindsay, W.L., and W.A. Norvell. 1978. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal, 42, 421–428.
Maas, E.V. 1986. Salt tolerance of plants. Applied Agricultural Research, 1(1), 12–26.
Maas, E.V., and S.R. Grattan. 1999. Crop yields as affected by salinity. In R.W. Skaggs and J. van Schilfgaarde (Eds.), Agricultural drainage, Agronomy Monograph No. 38. Madison, WI: ASA/CSSA/SSSA.
Minson, D.J. 1990. Forage in ruminant nutrition. San Diego, CA: Academic Press Inc.
Murphy, S., D. Giménez, L. Muldowney, and J. Heckman. 2012. Soil organic matter level and interpretation [FS1136]. Rutgers: New Jersey Agricultural Experiment Station. Available at https://njaes.rutgers.edu/pubs/publication.asp?pid=FS1136
Olsen, S.R., and L.E. Summers. 1982. Phosphorus. In A.L. Page, R.H. Miller, and D.R. Keeney (Eds.), Methods of Soil Analysis, Part 2, 2nd ed. Madison, WI: ASA/CSSA/SSSA.
Parker, M.B., G.J. Gascho, and T.P. Gaines. 1983. Chloride toxicity of soybeans grown on Atlantic coast flatwoods soils. Agronomy Journal, 75, 439–442.
Pearson, G.A. 1960. Tolerance of crops to exchangeable sodium [Information Bulletin 216]. Washington, D.C.: U.S. Department of Agriculture.
Rhoades, J.D. 2012. Diagnosis of salinity problems and selection of control practices: An overview. In W.W. Wallender and K.K. Tanji (Eds.), Agricultural salinity assessment and management, 2nd ed. (p. 40). Reston, VA: American Society of Civil Engineers.
Richards, L.A. (Ed.). 1954. Diagnosis and improvement of saline and alkali soils [Agriculture Handbook No. 60]. Washington, D.C.: U.S. Department of Agriculture. Available at https://www.ars.usda.gov/sp2UserFiles/Place/20360500/hb60_pdf/hb60complete.pdf
Saxton, K.E., and W.J. Rawls. 2006. Soil water characteristics by texture and organic matter for hydrologic solutions. Soil Science Society of America Journal, 70, 1569–1578.
For more on this topic, see the following publications:
A-114: Test Your Garden Soil
https://pubs.nmsu.edu/_a/A114/
A-146: Appropriate Analyses for New Mexico Soils
https://pubs.nmsu.edu/_a/A146/
A-148: Understanding Soil Health for Production Agriculture in New Mexico
https://pubs.nmsu.edu/_a/A148/
CR-656: An Introduction to Soil Salinity and Sodium Issues in New Mexico
https://pubs.nmsu.edu/_circulars/CR656/

Robert Flynn is an Associate Professor of Agronomy and Soils and an Extension Agronomist at New Mexico State University. He earned his Ph.D. at Auburn University. His research and Extension efforts aim to improve grower options that lead to sustainable production through improved soil quality, water use efficiency, and crop performance.
To find more resources for your business, home, or family, visit the College of Agriculture and Home Economics on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
October 2015