Guide A-229
Natalie Goldberg and Phillip Lujan
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Respectively, Distinguished Extension Specialist Emeritus and Program Manager, Department of Extension Plant Sciences, New Mexico State University. (Print Friendly PDF)
Phymatotrichum root rot, also known as cotton root rot or Texas root rot, is caused by the soil-borne fungus Phymatotrichopsis omnivora. Of all diseases known to occur on broadleafed plants, this is one of the most destructive and difficult to control. The pathogen has an incredibly wide host range and surefire survival techniques—two features that make management a nightmare for unfortunate growers faced with the disease. Although thoroughly studied since its discovery in 1888, researchers, farmers, and homeowners still have little ammunition in the fight against the disease.
Fortunately, this fungus is limited in distribution and does not readily spread from one location to another. The fungus is restricted geographically to the southwestern United States and northern Mexico in calcareous, alkaline soils with a pH range of 7.0 to 8.2 and with low organic matter. It also occurs only at elevations below 5,000 feet and is typically found in relatively small areas. In New Mexico, it occurs only in the southern counties, and is most prevalent in agricultural areas along the Rio Grande and Pecos rivers. It spreads slowly from plant to plant when a fungal strand from an infected root grows through the soil to a nearby healthy root. It has no means of airborne spread.
|
Diagnosis at a Glance |
|
|
Causal agent |
Soil-borne fungus, Phymatotrichopsis omnivora |
|
Hosts |
Over 2,300 species of broadleafed plants |
|
Symptoms |
Slight yellowing and bronzing of leaves |
|
Wilt |
|
|
Reddish lesion on crown |
|
|
Rapid death (leaves remain firmly attached) |
|
|
Signs |
Fungal threads on roots |
|
Spore mats around infected plants |
|
|
Sclerotia |
|
|
Conditions for disease |
Alkaline soils (pH 7.0–8.2) |
|
Soils low in organic matter |
|
|
High summer temperatures |
|
|
Management |
Avoid infested locations |
|
Plant non-hosts or resistant species |
|
|
Alter the soil environment |
|
The fungus has unique biological characteristics that contribute to management difficulties. First, P. omnivora has an extremely wide host range, infecting over 2,300 species of unrelated dicotyledonous (broadleafed) plants. The fungus attacks only mature plants; seedlings are not susceptible to the disease. In New Mexico, this is an important disease of cotton, alfalfa (Figure 1), grapes, fruit trees, nut trees, and many ornamentals, including conifers. The fungus can also survive on roots of native vegetation, such as mesquite, without causing any disease. Second, the fungus persists almost indefinitely in soil. Much of the fungus is found in the top 2 to 6 feet of soil; however, sclerotia (fungal survival structures; Figure 2) have been found over 12 feet deep. Another concern is that isolates of the fungus are non-specific. For example, an isolate infecting cotton is also virulent on fruit trees and ornamentals and vice versa. Thus, housing developments in old cotton and alfalfa fields with a history of the disease can be disastrous for urban landscapers.

Figure 1. Phymatotrichum root rot in alfalfa.
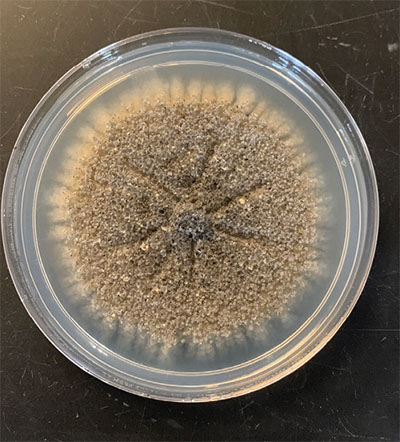
Figure 2. Sclerotia of P. omnivora.
The fungus is only active in summer months when air and soil temperatures are high. The greatest incidence of disease occurs when soil temperature at 1 foot deep is greater than 80°F and the air temperature in the plant canopy is above 104°F. When environmental conditions are favorable for fungal activity, the pathogen invades plants through their root systems. Infected roots rot and cannot transport water to the aboveground portion of the plant. Symptoms on aboveground plant parts resemble water stress. The first evidence of disease is a slight yellowing of the leaves. Leaves quickly turn to a bronze color and begin to wilt. Permanent wilting of the branches can occur very rapidly, as little as two weeks from the first expression of disease. The tree dies with leaves remaining firmly attached (Figure 3). In some cases, the tree wilts so quickly that the leaves hardly change color, though they will become dry and brittle. A reddish lesion around the crown of the plant may develop on trees killed by the fungus.

Figure 3. Tree killed by P. omnivora.
Evidence of P. omnivora can also be found on or near infected trees. The pathogen produces fungal strands on the surface of infected roots (Figure 4). These strands look like threads and are visible with a good hand lens. When strands are observed under a compound microscope, cruciform (cross-shaped) hyphae with pointed terminals unique to this fungus can be seen (Figure 5). Another sign is the formation of a white- to tan-colored spore mat on the surface of the soil around infected plants (Figure 6). Spore mats develop during periods of high moisture. The spores in these mats have never been germinated and are considered to have no function in survival or infection of the pathogen. Therefore, spore mats do not spread disease, but are evidence of its presence.

Figure 4. Fungal strands on infected roots.

Figure 5. Characteristic cruciform hyphae.
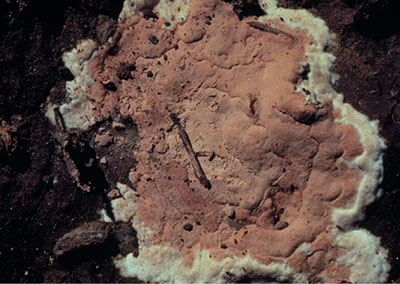
Figure 6. Spore mat.
Research to control this disease has been extensive, yet there are no good control methods. There is no resistance or tolerance to this disease in most of the commonly infected hosts. The best recommendation is to avoid land known to be infested with the fungus. Furthermore, fruit and nut orchards should not be planted in old cotton or alfalfa fields. If mesquite land is to be cleared for orchard planting, it may be worth the time and money to preplant the area with indicator plants like cotton. Plants with a tap root like this are infected and show symptoms in one season, while trees with fibrous root systems do not show disease for some time after planting. If no symptoms develop on this test crop, growers can be relatively sure the land is safe for growing ornamentals or fruit or nut trees.
The ability of the fungus to survive deep in the soil has eliminated the possibility of using fungicides and fumigants to control the disease because these materials can only penetrate a limited distance into the soil. Fumigating infested planting holes in areas where trees have died will usually only delay the onset of disease in newly planted trees. When a replanted tree’s roots reach deep enough in the soil, they will contact the surviving fungus and succumb to the disease.
There are practices that can be used to try to alter the soil environment so it no longer favors P. omnivora. These practices may help reduce the effect of the pathogen if they are followed every year. If not, the soil environment returns to its typical state (high pH and low organic matter) and again favors the fungus. The procedure consists of loosening the soil in a broad and comparatively shallow basin just beyond the drip line around infected trees. The area is then covered to a depth of 2 inches with manure or similar organic matter. Layered on top of the organic matter is ammonium sulfate and sulfur, each at a rate of 1 lb/10 sq ft. The basin should be immediately flooded with enough water to wet the soil to a depth of 3 feet. This high level of soil moisture must be maintained for several weeks. If trees are treated before permanent wilting, they may recover. Known root rot-infested areas should be treated every year in March or April.
Some success in reducing the effect of the disease has been achieved by growing and incorporating a green manure cover crop over the orchard floor. This helps to stimulate vigorous rooting of the trees, enabling them to better withstand disease pressure. Additionally, incorporating the cover crop into the soil may help to stimulate soil microflora, which compete with P. omnivora.
Finally, chemical barriers have been used to prevent the spread of disease from one tree to another. Using sulfur in trenches 4 to 6 inches wide and 4 to 6 feet deep around the outside of the drip line of infected trees has prevented the spread of P. omnivora for over 7 years.
In areas known to be infested with P. omnivora, the only sure control is to plant non-hosts. Unfortunately, the extensive host range of the pathogen limits choices. Monocots, such as corn, small grains, and all grasses, are all immune. Dicots grown as annuals often escape the disease. Additionally, most native desert plants are apparently tolerant to the disease.
To sample for P. omnivora disease diagnosis by the New Mexico State University Plant Diagnostic Clinic, select roots that are discolored and appear to have the outer bark or cortical tissue partially rotted. Not all roots will have the fungus present, and therefore require several root samples must be collected. Roots should be at least 6 inches in length and at least 1/4 inch in diameter. Do not wash to remove soil from the sampled roots; rather, gently shake off loose soil. After sampling, store roots in a plastic bag under refrigeration until they are ready to be delivered to your local county Cooperative Extension Service Office (https://aces.nmsu.edu/county/) or shipped directly to the Plant Diagnostic Clinic. For more information on submitting plant specimens, see NMSU Extension Guide H-158, How to Collect and Send Plant Specimens for Disease Diagnosis (pubs.nmsu.edu/_h/H158/).
The best thing that can be said about this fungus is that it is not widespread. It is found only in small pockets and does not spread much from its point of origin. There are no viable spores to help spread the disease from one location to another. The only known spread between plants occurs when fungal threads from infected roots contact healthy roots.
For Further Reading
H-106: Curly Top Virus
pubs.nmsu.edu/_h/H106/
H-158: How to Collect and Send Plant Specimens for Disease Diagnosis
pubs.nmsu.edu/_h/H158/
H-242: Tomato Spotted Wilt Virus
pubs.nmsu.edu/_h/H242/

Natalie P. Goldberg is a Distinguished Extension Specialist Emeritus in the Department of Extension Plant Sciences at NMSU. She earned her B.S. in ornamental horticulture from Cal Poly Pomona and her M.S. and Ph.D. in plant pathology from the University of Arizona. Her Extension program focuses on plant health management, plant disease identification, and crop biosecurity.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu.
Contents of publications may be freely reproduced, with an appropriate citation, for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
Revised November 2020 Las Cruces, NM


