Hops Virus Testing- Significance and Implications for Establishing Hop Production in New Mexico and Southwest Colorado
Research Report 788
Kevin A. Lombard, Beth LaShell, Franklin J. Thomas, Jason French, and Todd Bates
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Associate Professor, Department of Plant and Environmental Sciences, Agricultural Science Center at Farmington, New Mexico State University (P.O. Box 1018, Farmington, NM 87499-1018; klombard@nmsu.edu); Old Fort at Hesperus Coordinator, Fort Lewis College, Durango, CO; Research Lab Technician, Agricultural Science Center at Farmington, NMSU; Program Manager, Department of Extension Plant Sciences, NMSU; and Independent Researcher (P.O. Box 24, Embudo, NM 87531). (Print friendly PDF)
Summary
Some agricultural producers in New Mexico and southwestern Colorado view hops (Humulus lupulus and H. lupulus var. neomexicanus), used in bittering and flavoring beer, as a potential specialty crop for local craft brewing needs. Regional trials in northwestern New Mexico and southwestern Colorado indicate adaptability of some cultivars to a high-altitude, high-desert climate, where diurnal temperature swings are extreme and soil pH can exceed 8. There have been reports, however, of viruses infecting rhizomes commonly used to establish hop yards, and this prompted an examination of potential plant infection by viruses in research plots located at the New Mexico State University Agricultural Science Center at Farmington (NMSU-ASC Farmington) and Ft. Lewis College Old Fort at Hesperus, CO, experimental farms. In 2014, hop rhizomes collected from research plots were tested for the presence of Apple mosaic virus (ApMV), American hop latent virus (AHLV), Strawberry latent ringspot virus (SLRSV), Tobacco necrosis virus (TNV), and Arabis mosaic virus (ArMV). In one study established in 2008 at the NMSU-ASC Farmington with non-certified virus-free material, 50% of ‘Cascade’ entries tested positive for ApMV and 17% were co-infected with ApMV and AHLV. Strawberry latent ringspot virus, Tobacco necrosis virus, and Arabis mosaic virus were absent in tested rhizomes. Certified virus-free and H. lupulus var. neomexicanus entries were free of the five viruses we tested for. Establishing hop yards in New Mexico and Colorado with certified virus-free rhizomes or plantlets is critical to avoid the risk of reduced yields and viral transmission into unaffected hop plantings.
Introduction
Craft breweries are defined by having an annual production of less than 6 million barrels of beer per year, being independently owned, and brewing with traditional or innovative ingredients (Brewers Association, 2015). United States craft brewing realized $14.3 billion in retail sales in 2013—a 20% growth in sales over 2012 (Brewers Association, 2014). An important raw ingredient used in brewing is hops (Humulus lupulus), whose cones (strobiles) are used for bittering and flavoring beer (Figure 1). The Pacific Northwest dominates U.S. hop production and processing, with Washington state (e.g., Yakima Valley) producing 79% of the U.S. crop, followed by Oregon and Idaho. Total value of the U.S. hop crop from these three states was $249 million in 2013 (USDA, 2013).
Hop cones are borne annually on 6 m (20 ft) tall bines (twining stems) that originate from long-lived perennial rhizomes (underground stems). Hop bines require extensive trellising systems and labor-intensive thinning and training to increase bine vigor and hop cone yield. Hops are easily propagated asexually (to produce clones) via rhizomes, which are used to multiply hop yards of uniform growth, cone harvest date, yield, and chemistry (Figure 1).
Many states have adopted “buy local” campaigns supporting regional agriculture (e.g., New Mexico–Grown with Tradition® and Colorado Proud™), and New Mexico and Colorado craft brewers may be interested in purchasing their hops from a local grower, as evidenced by recent workshops conducted in Farmington, NM, and Durango, CO (Rodebaugh, 2013). Previous research at the NMSU-ASC Farmington indicates that some standard hop cultivars grow well in northern New Mexico, even possessing certain terroir characteristics. For instance, in Farmington, NM, ‘Cascade’ attained 9.8% alpha acids and 6.1% beta acids, higher than values reported in the literature for the same cultivar (ten-year range: 5.1–8.5% alpha, 4.0–6.6% beta) when grown in the Pacific Northwest (Freshops, 2014; Lombard et al., 2014). Additionally, hop yards of New Mexico native H. lupulus var. neomexicanus are being viewed by some growers as a value-added cultivar that could be branded to produce craft beer specific to the Southwest region (Geiling, 2014; Merchant, 2013).

Figure 1. (A) Hop yard in Yakima, WA; (B) training hop bines at the NMSU-ASC Farmington, NM; (C) hop cones (strobiles), which produce bittering and aromatic compounds; and (D) hop rhizomes used to clonally propagate or multiply hop yards of uniform planting blocks.
Viruses In Hops
At least 12 viruses have been reported in hops, with Hop mosaic virus (HpMV), Hop latent virus (HpLV), Arabis mosaic virus (ArMV), American hop latent virus (AHLV), and Apple mosaic virus (ApMV) generally considered to be the five most economically important viruses (Pethybridge et al., 2008). Prunus necrotic ringspot ilarvirus (PNRV) is also an important hop virus in the U.S., while Alfalfa mosaic virus (AMV) (hop strain), Cherry leaf roll virus (CLRV), Humulus japonicus latent virus (HULV), and Petunia asteroid mosaic virus (PetAMV) are important viruses in Europe and Australia (Biosecurity Australia, 2010; Pethybridge et al., 2008; Postman et al., 2005).
Apple mosaic virus has a wide host range, infecting at least nine different plant families, and is generally regarded as having the greatest impact on yield among the commonly occurring viruses in hops (Pethybridge et al., 2008). Depending on cultivar, infection by ApMV can reduce cone weight by up to 50% and alpha acid content by up to 10% (Pethybridge et al., 2008). Sensitivity and loss to ApMV varies significantly among hop cultivar, location, and season. Symptoms of ApMV include mottled, chlorotic ringspots that eventually form necrotic, oak-leaf line patterns (Figure 2; Eastwell and Ocamb, 2014; Pethybridge et al., 2008). Symptom expression appears to depend heavily on growing conditions (with cooler temperatures expressing more symptoms than hotter temperatures or where there are rapid temperature fluctuations), hop cultivar, and virus concentration (Eastwell and Ocamb, 2014; Pethybridge et al., 2008).
American hop latent virus (genus Carlavirus) was first reported in 1976, and is found in United States and New Zealand hop yards and has been found and destroyed in quarantined material in Australia and Europe (Pethybridge et al., 2008). Consequences of infection appear to mimic changes associated with advanced age of plants, although no visual symptoms are reported in hop cultivar crowns in the Pacific Northwest (Eastwell and Ocamb, 2014). In susceptible cultivars, AHLV infection can decrease cone yield by up to 62% and decrease alpha acid content by up to 18% (Jelinek et al., 2012).
In most current commercially important hop cultivars, virus infection is symptomless, making detection and diagnosis difficult (Eastwell and Ocamb, 2014). In many hop yards, co-infection with multiple virus species naturally occurs, reducing yields in susceptible cultivars when little or no yield reduction is observed in the presence of an individual virus (Pethybridge et al., 2008). While yield reductions may not be observed in virus-
tolerant hop cultivars, the presence of infected plants can serve as an important reservoir for virus spread to non-infected hops. For example, ApMV, AHLV, and other hop viruses are spread in hop yards by plant-to-plant contact (root grafting and shoot contact); mechanically through cultural operations such as mowing, stringing, training, leaf stripping, and thinning; insects like damson hop aphid (Phorodon humuli), important in AHLV transmission; and by planting infected rhizomes (Pethybridge et al., 2008; Pethybridge et al., 2002). Clonally propagated plants like hops, grown intensely for long periods of time without isolation, are prone to the accumulation of viruses (Hartmann et al., 2002). As a result, there are substantial risks to hop yards using rhizomes from plantings of unknown virus infection status. This practice has serious implications for spreading viruses to previously uninfected locations throughout New Mexico and Colorado.
The objective of this study was to test for the presence of various viruses in rhizomes collected from all entries (certified and non-certified virus-free in H. lupulus and H. lupulus var. neomexicanus) planted in test plots at the NMSU-ASC Farmington and Ft. Lewis College Old Fort at Hesperus, CO, experimental farms (beginning in 2008 and 2009, respectively). As interest in hops increases in New Mexico and southwestern Colorado, the overall goal is to educate backyard and commercial growers in the region on the importance of obtaining certified disease-free material for establishing hop yards.

Figure 2. Apple mosaic virus infection in hops. (Photos by David Gent, USDA Agricultural Research Service, Bugwood.org.)
Materials and Methods
Plant Material, Test Plot Establishment, Cultural Practices, and Harvesting
NMSU-ASC Farmington Experimental Trials
The ASC Farmington experimental hop yard is located at an elevation of 1,720 m (5,643 ft; lat. 36°41’20.95”N, long. 108°18’45.56” W). Soil is classified as a Doak sandy loam (fine-loamy, mixed, mesic Typic Haplargid), with a pH above 8 and less than 1% organic matter (Keetch, 1980).
Hop cultivars (Table 1) were obtained as rhizomes from the United States Department of Agriculture (USDA) Agricultural Research Service (ARS) Hop Breeding & Genetics program (Corvalis, OR) and Todd Bates (independent researcher, Embudo, NM). Tissue culture plantlets were obtained in 2013 from Summit Labs (Fort Collins, CO). Unlike rhizomes, tissue culture starts are derived from cultured apical meristem cells and were certified virus-free.
From 2008–2014, five experimental trials were planted at the NMSU-ASC Farmington with 0.9 m (3 ft) spacing between plants and 3.6 m (12 ft) between rows (Figure 3). Trials established in 2008, 2009, and 2014 consisted of four plants per cultivar entry replicated three times. Trials established in 2010 were not replicated. The trial established in 2013 was planted with six plants per cultivar entry with plots replicated four times.
Hop bines were thinned and trained annually. Thinning involved hand pulling of excess bines or using a gas-powered string-line trimmer to eliminate the first flush of growth. Second growth flush was trained 4–6 bines per string to the 4.3 m (14 ft) tall trellis.
About once per month during the growing season, hand hoeing between plants and rototilling between rows was used to control weeds. Hop cones were hand harvested annually by cutting bines at the base with pruning shears, transporting bines indoors, and pulling cones by hand.
Fort Lewis College Experimental Trials
An experimental plot of one row was established in 2009 at the Old Fort at Hesperus, CO, experimental farm at an elevation of 2,316 m (7,600 ft; lat. 37°13’46.2”N, long. 108°03’09.7”W), with between-plant spacing of 0.9 m (3 ft) and trained to a trellis approximately 4.3 m (14 ft) high. Hop cultivars tested (Table 1) were obtained as rhizomes from a hop trial planted in 2002 at the Colorado State University Western Colorado Research Center (Hotchkiss, CO) using rhizomes from various Pacific Northwest nurseries, although no other information was given on rhizome origin (Godin, personal communication). Cultural and harvesting practices were similar to those described for the NMSU-ASC Farmington.
| Table 1. Hop Cultivar Sampled, Year of Plot Establishment, Plot Row (refer to Figure 3), Certification Status of Plant Material, Source of Plant Material, and Number of Plants Sampled Per Entry | |||||
Cultivar |
Trial establishment year |
Plot row |
Certified virus-free | Source |
Number of plants sampled |
| NMSU-ASC Farmington trials | |||||
| ‘Cascade’ | 2008 | 1 | No | USDA | 6 |
| ‘Columbus’ | 2008 | 1 | No | USDA | 6 |
| ‘Crystal’ | 2008 | 1 | No | USDA | 6 |
| ‘Hallertauer’ | 2008 | 1 | No | USDA | 6 |
| ‘Newport’ | 2008 | 1 | No | USDA | 6 |
| ‘Northern Brewer’ | 2008 | 1 | No | USDA | 6 |
| ‘Centennial’ | 2009 | 2 | No | USDA | 6 |
| ‘Fuggle’ | 2009 | 2 | No | USDA | 6 |
| ‘Galena’ | 2009 | 2 | No | USDA | 4 |
| ‘Horizon’ | 2009 | 2 | No | USDA | 6 |
| ‘Nugget’ | 2009 | 2 | No | USDA | 4 |
| ‘Saaz’ | 2009 | 2 | No | USDA | 4 |
| ‘Sterling’ | 2009 | 2 | No | USDA | 6 |
| 10 unnamed H. lupulus var. neomexicanus selectionsz | 2010 | 3 | No | Todd Bates | 1–2 |
| ‘Cascade’ | 2013 | 4 | Yes | Summit Labs | 2 |
| ‘Centennial’ | 2013 | 4 | Yes | Summit Labs | 2 |
| ‘Crystal’ | 2013 | 4 | Yes | Summit Labs | 2 |
| ‘Nugget’ | 2013 | 4 | No | Summit Labs | 2 |
| ‘Latir’ | 2014 | 4 | No | Todd Bates | 2 |
| ‘Multihead’ | 2014 | 4 | No | Todd Bates | 2 |
| Fort Lewis College trials | |||||
| ‘Magnum’ | 2009 | 1 | No | Unknowny | 3 |
| ‘Chinook’ | 2009 | 1 | No | Unknown | 3 |
| ‘Nugget’ | 2009 | 1 | No | Unknown | 3 |
| ‘Red Vine’ | 2009 | 1 | No | Unknown | 2 |
| zEntries were in un-replicated plots. Two to three rhizomes were sampled per entry. Only 1–2 plants from each entry were sampled. yFort Lewis College trials were planted using rhizomes transplanted from a hop trial established in 2002 at the CSU Western Colorado Research Center (Hotchkiss, CO). The CSU trial was established using rhizomes from various Pacific Northwest nurseries; no other information given (Godin, personal communication). |
|||||
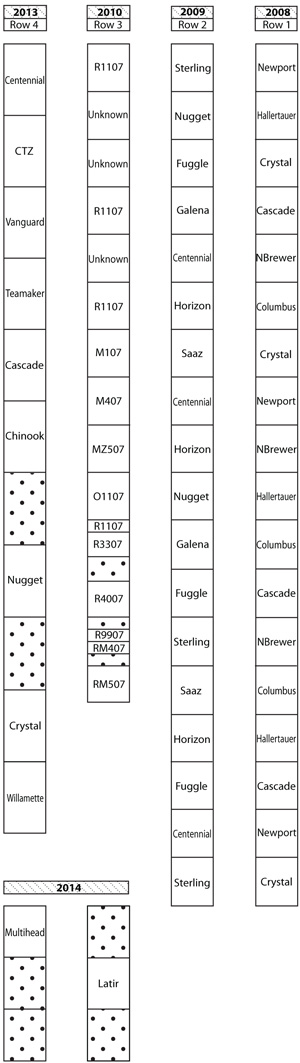
Figure 3. Configuration of NMSU-ASC Farmington hops (Humulus lupulus) trials planted 2008–2014. Note: There are 7 rows total, but only rows 1–4 were sampled.
Plant Material Tested
Rhizome samples were pulled from every plot in rows 1–4 in the NMSU-ASC Farmington trial (Figure 3) and from the single row planted at the Fort Lewis College trial (data not shown). At both trial locations, hop rhizomes (2–3 rhizomes from the same plant to achieve enough sample material volume) were dug from the inner 1–2 plants per entry plot. Rhizomes were placed into 1-gallon zip-lock bags, sealed, and sent overnight to the NMSU Plant Diagnostic Clinic (Las Cruces, NM) for virus testing. For the NMSU-ASC Farmington trials, a total of 6 plants were sampled per cultivar entry in rows 1 and 2, except for ‘Galena’, ‘Nugget’, and ‘Saaz’, where only 4 plants were sampled. For entries in rows 3 and 4, only 1–2 plants per cultivar entry were sampled. For the Fort Lewis College trial, 3 plants per cultivar entry were sampled, except for ‘Red Vine’ in which only 2 plants were sampled.
Laboratory Diagnostics
Given the large number of viruses reported to infect hops (Pethybridge et al., 2008) and the cost/availability of test kits, as a start, we tested rhizomes only for Apple mosaic virus (ApMV), American hop latent virus (AHLV), Strawberry latent ringspot virus (SLRSV), Tobacco necrosis virus (TNV), and Arabis mosaic virus (ArMV) by enzyme linked immunosorbent assay (ELISA) using kits purchased from AC Diagnostics (ACD; Fayetteville, AR). Rhizome tissue was ground at a 1:100 ratio of tissue weight to ACD’s extraction buffer volume, and the plates were processed according to manufacturer recommendations. Test results were examined visually; the development of a yellow color in a test well indicated a positive test result.
Data Analysis and Reporting
Data are descriptive and expressed as percentages of samples that tested positive for each virus in relation to the total number of samples submitted for each cultivar entry.
Results
NMSU-ASC Farmington Studies
Spatially, only rhizomes that were sampled from rows 1 and 2 tested positive and only ApMV and AHLV were detected in rhizomes; certified virus-free plants and H. lupulus var. neomexicanus planted in the remaining rows were virus-free. Row 1, established in 2008 (Figure 3), was most infected. ApMV infection was highest in ‘Hallertauer’ (100%), followed by ‘Columbus’ (67%), ‘Cascade’ (50%), and ‘Crystal’ (17%). AHLV was found in 17% of ‘Columbus’ samples. ‘Cascade’ and ‘Crystal’ had rhizomes that were co-infected with ApMV and AHLV. In row 2, established in 2009, ‘Galena’ was co-infected with both ApMV and AHLV, while the remainder of row 2 was virus-free (Table 2).
Fort Lewis College Study
All ‘Red Vine’ rhizomes were infected with ApMV, while ‘Chinook’, ‘Magnum’, and ‘Nugget’ were infected with AHLV (33%, 67%, and 67%, respectively; Table 2). Virus co-infection was not observed.
| Table 2. Percent Virus Infection by Trial Establishment Year for Apple mosaic virus (ApMV), Carlavirus American hop latent virus (AHLV), Strawberry latent ringspot virus (SLRSV), Tobacco necrosis virus (TNV), and Arabis mosaic virus (ArMV)* | ||||||
| Trial establishment year |
% Infected | |||||
| ApMV | AHLV | Co-infection ApMV and AHLV |
SLRSV | TNV | ArMV | |
| NMSU-ASC Farmington trials | ||||||
| 2008/Row 1 | ||||||
| ‘Cascade’ | 50 | 0 | 17 | 0 | 0 | 0 |
| ‘Columbus’ | 67 | 17 | 0 | 0 | 0 | 0 |
| ‘Crystal’ | 17 | 0 | 33 | 0 | 0 | 0 |
| ‘Hallertauer’ | 100 | 0 | 0 | 0 | 0 | 0 |
| ‘Northern Brewer’ | 0 | 0 | 0 | 0 | 0 | 0 |
| ‘Newport’ | 0 | 0 | 0 | 0 | 0 | 0 |
| 2009/Row 2 | ||||||
| ‘Centennial’ | 0 | 0 | 0 | 0 | 0 | 0 |
| ‘Fuggle’ | 0 | 0 | 0 | 0 | 0 | 0 |
| ‘Galena’z | 0 | 0 | 25 | 0 | 0 | 0 |
| ‘Horizon’ | 0 | 0 | 0 | 0 | 0 | 0 |
| ‘Nugget’z | 0 | 0 | 0 | 0 | 0 | 0 |
| ‘Saaz’z | 0 | 0 | 0 | 0 | 0 | 0 |
| ‘Sterling’ | 0 | 0 | 0 | 0 | 0 | 0 |
| Fort Lewis College trials | ||||||
| ‘Magnum’y | 0 | 67 | 0 | 0 | 0 | 0 |
| ‘Chinook’y | 0 | 33 | 0 | 0 | 0 | 0 |
| ‘Nugget’y | 0 | 67 | 0 | 0 | 0 | 0 |
| ‘Red Vine’x | 100 | 0 | 0 | 0 | 0 | 0 |
| *Only studies with detected virus infection are reported. Unless noted, sample size per entry was six (n = 6) samples per cultivar. Refer to Figure 3 for row location (NMSU-ASC Farmington trial only). zsample size n = 4 rhizomes tested per entry ysample size n = 3 rhizomes tested per entry xsample size n = 2 rhizomes tested per entry |
||||||
Discussion
While it is conceivable that virus transmission between entries could have spread via insect vectors, mechanical weeding, leaf stripping, thinning, and harvesting operations, the source of virus infection in tested rhizomes was most likely the non-certified disease-free rhizomes received in 2008 and 2009 at the time the studies in Farmington, NM, and Hesperus, CO, were established, respectively. Vegetative propagation of infected rhizomes and stems, especially in areas where hops are not intensely grown (like New Mexico and southwestern Colorado), remains one of the most important means of increasing the spread of virus infection in hop yards (Pethybridge et al., 2008). In fact, of 200 hop clones tested from the USDA-ARS National Clonal Germplasm Repository (NCGR; Corvallis, OR), 98 entries tested positive for one or more viruses, 50% of which tested positive for AHLV infection and 29% tested positive for ApMV infection, the two viruses that tested positive in the NMSU-ASC Farmington experimental plots (Postman et al., 2005). The USDA-ARS NCGR has since eliminated viruses from most of their Humulus collection through tissue culture methods (Postman et al., 2005). It is unclear why virus co-infection was only observed in the NMSU-ASC Farmington trials and not in the Fort Lewis College study. Insect scouting during the growing seasons suggests that damson hop aphid (P. humuli), which vectors AHLV, was absent in Farmington, NM, and Hesperus, CO. In New Mexico, the presence of damson hop aphid has not been observed, probably because of the low amount of hop plantings currently in the state (Grasswitz, personal communication).
Susceptibility to virus infection and expression of disease symptoms can be cultivar-specific (Pethybridge et al., 2008). Of the cultivars trialed at the NMSU-ASC Farmington, ‘Cascade’ has been one of the top performers (Lombard et al., 2014) despite being infected with ApMV singularly or co-infected with ApMV and AHLV. ‘Cascade’ entries appeared to be symptomless. On the other hand, ‘Hallertauer’, a noble hop cultivar from Germany, has been one of the poorest performers in Farmington. On high-pH soils found at the NMSU-ASC Farmington, ‘Hallertauer’ often exhibited symptoms of iron deficiency (Lombard et al., 2014). While ‘Hallertauer’ responded to applications of FeEDDHA chelated iron (Lombard, data unpublished), the presence of ApMV may have contributed to its overall poor performance.
Infected rhizomes of standard H. lupulus cultivars imported into New Mexico and southwestern Colorado could serve as a reservoir for viruses that could in turn be transmitted to uninfected hop yards of both standard H. lupulus and H. lupulus var. neomexicanus cultivars. To avoid inadvertent introduction of viruses into previously uninfected hop yards, Brown and Sirrine (2012) recommend that growers purchase certified disease-free stock material from a reputable and licensed dealer. The department of agriculture in the state where stock plant material originates can be contacted if there are questions about whether a nursery is licensed (Brown and Sirrine, 2012).
In terms of the limitations of this study, we ideally would have tested for all viruses and viroids reported in hops. Viroids are smaller than viruses, consisting of a single strand of RNA (Hartmann et al., 2002). Hop stunt viroid (HSVd) and other viroids are reported as economically important in hops (Pethybridge et al., 2000). Since this survey was conducted, all studies established in 2008 and 2009 have been eliminated and subsequent plots of standard cultivars have been established with certified disease-free plant material. A comprehensive virus and viroid survey is needed for the remaining H. lupulus var. neomexicanus plots.
Conclusion
Management of ApMV, AHLV, and other viruses relies solely on removing infected plants and replacing them with certified virus-free stock. Yard reestablishment when infection levels are high can be costly, and the benefits need to be weighed against cost since individual cultivars can vary markedly in susceptibility and tolerance to infection. While some virus-infected cultivars may be asymptomatic and yield normally, in New Mexico and Colorado, eliminating virus-infected plants will ensure that viruses do not spread to cultivars susceptible to yield loss or to H. lupulus var. neomexicanus native hops.
Acknowledgments
We thank Colorado and New Mexico Departments of Agriculture (Specialty Crop Block Grants), and New Mexico State University Agricultural Experiment Station and Fort Lewis College for funding and salary support. We thank John Henning of the USDA-ARS Hop Breeding & Genetics program and Ron Godin of Colorado State University Cooperative Extension for their assistance in establishing the Farmington, NM, and Fort Lewis College studies.
Additional Resources
Information about hop trials in northwestern New Mexico and southwestern Colorado
What’s Hop’n: A symposium on hops (Humulus sp.) production and marketing in the Four Corners Region and New Mexico
Archived presentations from a symposium held July 12–13, 2013 (http://aces.nmsu.edu/hch/hopsresearch.html). Participants included a combination of those thinking about growing hops and those thinking of commercially brewing with fresh/non-pellet hops. The emphasis was placed on commercial, small-scale farm production and the pros and cons of growing and marketing hops.
2013 high-altitude hop variety research trial at the Old Fort at Hesperus, CO
- Variety descriptions
http://www.fortlewis.edu/Portals/178/HopsVarieties-Old%20Fort.pdf - Hop yard history and establishment
http://www.fortlewis.edu/Portals/178/Old%20Fort%20Hops%20Yard%20History%20and%20Establishment.pdf
Description of USDA and industry named hop cultivars
Freshops. 2014. USDA named hop variety descriptions. Oregon State University High Alpha Acid Breeding Program.
Hop Growers of America. 2011. Variety manual: USA hops.
Tissue culture starts for certified virus-free hops
Summit Plant Laboratories
Phone: (970) 224-2021 / (800) 654-1017
http://www.plantlabs.com/clean-stocks/hop-field-transplants/
Literature Cited
Biosecurity Australia. 2010. Final review of policy: Importation of hop (Humulus species) propagative material into Australia.
Brewers Association. 2014. Brewers Association announces 2013 craft brewer growth. Retrieved September 3, 2014, from http://www.brewersassociation.org/press-releases/brewers-association-announces-2013-craft-brewer-growth/
Brewers Association. 2015. Craft brewer defined. Retrieved April 6, 2015, from https://www.brewersassociation.org/statistics/craft-brewer-defined/
Brown, D., and R. Sirrine. 2012. Purchasing hops for planting. Michigan State University Extension. Retrieved June 27, 2014, from http://msue.anr.msu.edu/news/purchasing_hops_for_planting
Eastwell, K., and C.M. Ocamb. 2014. Hop (Humulus lupulus)-virus diseases. Pacific Northwest Plant Disease Management Handbook.
Freshops. 2014. USDA named hop variety descriptions. Retrieved June 17, 2014, from http://freshops.com/hops/usda-named-hop-variety-descriptions
Geiling, N. 2014. In search of the great American beer. Smithsonian. Retrieved July 30, 2014, from http://www.smithsonianmag.com/arts-culture/search-great-american-beer-180951966/?no-ist
Hartmann, H.T., D.E. Kester, F.T. Davies, Jr., and R.L. Geneve. 2002. Plant propagation: Principles and practices, 7th ed. Upper Saddle River, NJ: Prentice Hall.
Hop Growers of America. 2011. Variety manual: USA hops. Moxee, WA: Author. Retrieved June, 26, 2014.
Jelinek, L., M. Doleckova, M. Karabin, T. Hudcova, B. Kotlikova, and P. Dostalek. 2012. Influence of growing area, plant age, and virus infection on the contents of hop secondary metabolites. Czech Journal of Food Science, 30, 541–547.
Keetch, C.W. 1980. Soil survey of San Juan County New Mexico: Eastern part. Washington, D.C.: U.S. Department of Agriculture.
Lombard, K.A., R. Acharya, F.J. Thomas, and K. McCarver. 2014. Hops (Humulus lupulus) evaluation. In M.K. O’Neill (Ed.), 2013 Annual Progress Report, Agricultural Science Center at Farmington (pp. 122–126). Farmington, NM: New Mexico State University Agricultural Experiment Station. Available at http://farmingtonsc.nmsu.edu/documents/NMSU%20AnnRpt%202013%20All%20MKON%20-%20final%20(reduced%20size).pdf
Merchant, B. 2013. Growing & harvesting monastery hops: Native New Mexican varieties. Paper presented at the WHAT’s HOP’N: A symposium on hops (Humulus sp.) production and marketing in the Four Corners Region and New Mexico, Farmington, NM. Retrieved September 19, 2014, from http://aces.nmsu.edu/hch/hopsresearch.html
Pethybridge, S.J., C.R. Wilson, L.J. Sherriff, G.W. Leggett, and D. Munro. 2000. Virus incidence in Australian hop (Humulus lupulus L.) gardens and cultivar differences in susceptibility to infection. Australian Journal of Agricultural Research, 51, 685–690.
Pethybridge, S.J., C.R. Wilson, F.S. Hay, G.W. Leggett, and L.J. Sherriff. 2002. Mechanical transmission of Apple mosaic virus in Australian hop (Humulus lupulus) gardens. Annals of Applied Biology, 141, 77–85.
Pethybridge, S.J., F.S. Hay, D.J. Barbara, K.C. Eastwell, and C.R. Wilson. 2008. Viruses and viroids infecting hop: Significance, epidemiology, and management. Plant Disease, 92, 324–338.
Postman, J.D., J.S. DeNoma, and B.M. Reed. 2005. Detection and elimination of viruses in USDA hop (Humulus lupulus) germplasm collection. Paper presented at the 1st International Symposium on Humulus. Acta Horticulturae, 668, 143–148.
Rodebaugh, D. 2013, July 7. Time to hop to it? Durango Herald. Available from http://durangoherald.com/apps/pbcs.dll/article?AID=/20130711/NEWS01/130719883/-1/News01/Time-to-hop-to-it&-&template
USDA–NASS. 2013. 2013 hop production up thirteen percent from last year.
For more on this topic, see the following publications:
H-158: How to Collect and Send Plant Specimens for Disease Diagnosis
https://pubs.nmsu.edu/_h/H158/index.html
CR-573: Drip Irrigation for Row Crops
https://pubs.nmsu.edu/_circulars/CR573/index.html
All Horticulture Publications
https://pubs.nmsu.edu/_h/

Kevin A. Lombard is an Associate Professor of horticulture with split appointments at New Mexico State University’s Agricultural Science Center at Farmington and San Juan College (Farmington, NM). He earned his Ph.D. from New Mexico State University. His research focuses on evaluating the intersections of specialty horticultural crops with community health and livelihood enhancement, with a focus on serving the residents of the Four Corners Region.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at aces.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
July 2015


