Guide H-329
Revised by Gill Giese and Phillip Lujan
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Extension Viticulture Specialist and Program Manager, Department of Extension Plant Sciences, New Mexico State University. (Print Friendly PDF)
Scope and Symptoms
Powdery mildew disease, caused by the fungus Erysiphe necator (formerly Uncinula necator), afflicts grape-growing regions worldwide, including New Mexico. The disease reduces foliar photosynthesis, causes premature leaf drop, reduces crop yields and quality, and, when left unmanaged over multiple years, predispose the buds and canes to cold injury and can lead to premature vine death. Grape berries infected with powdery mildew may develop skin cracks and are more susceptible to infection by Botrytis bunch rot and spoilage organisms that severely compromise fruit quality. As little as 3–5% powdery mildew-infected grape berries at harvest are detrimental to wine quality.
Symptoms are mostly observed during New Mexico’s “monsoon” season in late summer, although infection frequently occurs earlier in the season. Frequent rain showers, overcast skies that limit the canopy’s exposure to UV light and radiative heat, relatively moderate temperatures, and relative humidity above 75% provide ideal conditions for infection and spread of the fungus. The disease is easily recognized by a dusty white-gray or greenish-white coating on leaves or other above-ground green plant parts, and is commonly observed on the upper surfaces of leaves (Figures 1A and 1B). It can also affect lower leaf surfaces, young green shoots, buds, flowers, and fruit (Figures 2 and 3). Infections that were active when the shoot was green during the growing season can leave “scars” on dormant brown canes (Figures 4A and 4B). Severely infected leaves may exhibit mottling or deformity, including leaf curling and withering (Figure 5). Initially, infected fruit turn grayish-white, develop a brown and rusted appearance, and may crack, shrivel, or drop from clusters.
Figures 1A and 1B. Powdery mildew-infected leaf early in the season (A) (A. Baudoin, Virginia Tech), and a leaf with advanced powdery mildew late in the growing season (B) (G. Giese, NMSU Cooperative Extension).
Figure 2. Recently set fruit infected with powdery mildew (G. Giese, NMSU Cooperative Extension).
Figure 3. Severely infected berries with some cracking evident. This level of disease indicates a complete loss of disease control. The fruit is not recoverable within the current growing season (G. Giese, NMSU Cooperative Extension).
Figures 4A and 4B. A growing green grapevine shoot with active powdery mildew infection sites (A) (A. Baudoin, Virginia Tech). Dormant grape canes with discolored “scars”; darker regions are due to powdery mildew infections in the previous growing season (B). These “scars” are not viable powdery mildew. Grapevines have indeterminate growth; the scarring location on the cane can indicate when, during the previous growing season, powdery mildew was present (G. Giese, NMSU Cooperative Extension).
Figure 5. Advanced, late-season (post-harvest) powdery mildew-infected grapevine canopy indicating total loss of disease control (Sandoval County, NM), with deformed, yellowing, and withered leaves. No fungicide applications were made the entire season. These vines were likely infected early, prior to harvest, and were left untreated going into dormancy (G. Giese, NMSU Cooperative Extension).
Life Cycle and Conditions for Disease
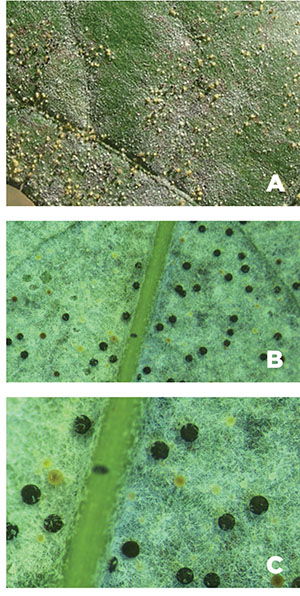
The powdery mildew fungus overwinters as hyphae inside dormant buds, or as chasmothecia (spore-bearing structures) in bark crevices of semi-permanent trunks and cordons, and on infected fruit and leaves. (Figures 6A–6C). The chasmothecia detach from the bearing colony, and are easily wind-dispersed or washed away; thus, they need loose bark or crevices for lodging.
Figures 6A–6C. Chasmothecia (overwintering form of powdery mildew) established and in various stages of maturity (A) on an infected grape leaf (A. Baudoin, Virginia Tech). Mature chasmothecia are black in color (B and C) (P. Lujan, NMSU).
When hyphae originating from dormant buds serve as the primary inoculum, the new green leaf tissue is infected when the bud breaks dormancy. When chasmothecia provide the primary inoculum, plants are infected in the spring as ascospores (sexual spores) are released from the overwintering structures. The release of ascospores requires the presence of free moisture in the form of rain, overhead irrigation, or heavy fog or dew. Ascospores shoot up into the air currents and are wind-blown to susceptible plant tissue, where new infections can occur at temperatures above 50°F with as little as 0.1 inch of rain or overhead irrigation. The fungus produces conidia (asexual spores) during the growing season that increase the severity of the disease on infected plants and may spread the fungus from one plant to another. Powdery mildew is favored by temperatures between 68 and 85°F and relative humidity between 60 and 90%. After the ascospore release in spring, “free” moisture in the form of dew or raindrops (liquid water), as opposed to water vapor, is detrimental to the organism. This explains why the disease continues to occur under relatively dry conditions.
Low, diffuse light and reduced air circulation in the interior of a dense grapevine canopy favor conidial survival and disease. These conditions, coupled with overcast skies and relatively high humidity in general, favor fungal sporulation and growth. For this reason, powdery mildew infections are often worse in dense canopies where shade and minimal air circulation prevail. Keep in mind that powdery mildew can occur at any time during the growing season and is likely present but unobserved early in the season.
Management
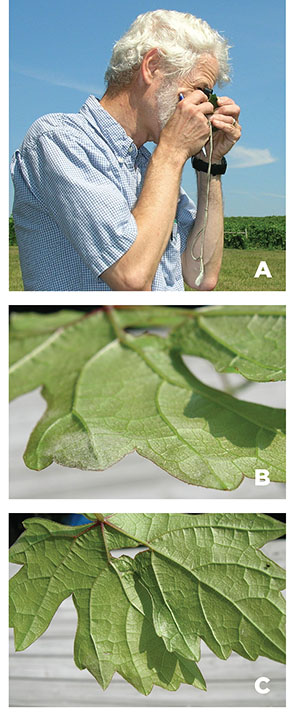
Powdery mildew disease management is about prevention rather than eradication of existing disease. Once the vine is infected, damage has been done and cannot be reversed in-season. Effective and timely scouting for early detection of the disease can aid in its management (Figures 7A–7C). Proactive cultural management includes selecting resistant cultivars and planting locations that have good airflow because grapevine canopies at these locations will dry faster. Although the degree of susceptibility to powdery mildew among Vitis vinifera cultivars differs, most are debilitated when the disease is left unchecked. However, many hybrid cultivars offer a greater degree of powdery mildew resistance. Grape breeding programs continue to develop and release new hybrids with genetic resistance to powdery mildew as well as high-quality winemaking attributes.
Figures 7A–7C. Inspecting leaves with the sun over your shoulder and a hand lens to provide magnification (A), and turning a leaf at an angle to make infections easier to see (B and C). Infections can occur on either side of the leaf (A. Baudoin, Virginia Tech).
Avoid crowding vines together when planting or training. Strategic pruning, trellising, and canopy management can increase light penetration and ventilation to reduce relative humidity within the vine canopy. Airflow and ventilation discourage mildew infection and growth. A relatively high trellised canopy designed with air ventilation in mind is preferable to a canopy with restricted ventilation and high leaf density. Selectively pruning overcrowded plantings and removing leaves increases light and air circulation, which decreases relative humidity within the grapevine canopy and allows for more effective spray penetration. However, leaf removal should be judicious and occur well before veraison. Generally, leaf removal is best done during the time interval from full bloom until berries are “peppercorn to pea-size,” or 4–7 mm in diameter, between E-L phenological growth stages 23 and 31. This relatively early timing of leaf removal allows fruit clusters and the vine canopy to acclimate prior to grape ripening. However, with increased sunlight exposure, there is increased risk of sunburn and direct damage to ripening fruit clusters (Figure 8).
Figure 8. Sunburned, pre-veraison grape cluster due to overexposure (G. Giese, NMSU Cooperative Extension).
Avoid nitrogen fertilizer applications after early July to limit growth of succulent vegetative tissue late in the growing season. Most New Mexico grape growers use drip or flood irrigation, and it is recommended to avoid overhead watering that can increase relative humidity. Water early in the morning to let the plant tissue and soil dry as quickly as possible. Infected plant debris can be safely composted because the powdery mildew organism is an “obligate biotroph” that requires living tissue to grow and reproduce.
Fungicides can be used for managing powdery mildew. Although overwintering fungus becomes active and can infect new growth, usually the best time to scout and initiate fungicide application is when new shoots are about 3 inches long. The first few fungicide treatments are the most important and should be applied at appropriate intervals according to the fungicide label and prevailing weather conditions. A powdery mildew index (PMI) model can inform appropriate treatment intervals since frequency of treatment depends upon weather conditions and choice of fungicide. For more information on calculating PMI, please see the University of California’s Agriculture and Natural Resources integrated pest management program at www.ipm.ucdavis.edu. The site is designed to help monitor and interpret climate indices that would preclude fungicide treatments.
Many different fungicides are labeled to help manage powdery mildew, and a partial listing is provided in Table 1. Powdery mildew fungicides are classified into different groups based on their mode of action. This classification is referred to as their FRAC groups (Fungicide Resistance Action Committee). For best results, rotate fungicides with different FRAC group numbers; the exception to this recommendation are fungicides that have an “M” (multisite) or “NC” (not classified) FRAC code. These fungicides are at very low risk for resistance development. Rotating and tank mixing fungicides from different FRAC groups is the best approach for preventing or slowing the development of resistance to fungicides. One should also avoid relying on fungicides for which resistance is known to be prevalent in the region. Fungicide recommendations change frequently, and growers are encouraged to refer to current guides. For more information on managing fungicide resistance, refer to Moyer and Grove (2012) and at https://framenetworks.wsu.edu/.
|
Table 1. Selected Fungicides with Activity on Powdery Mildew |
|||||
|
Fungicide chemical name/ Trade name/ Manufacturer name |
FRAC Group |
Toxicity |
Activity |
Resistance risk |
Restrictions/notes |
|
difenoconazole + cyprodinil Inspire Super Syngenta |
3, 9 |
Caution |
+/- protectant post-infection anti-sporulant |
high |
Do not apply more than 0.46 lb difenoconazole or more than 1.4 lb cyprodinil/acre/year. May cause leaf burn in non-vinifera varieties. |
|
quinoxyfen Quintec Corteva |
13 |
Caution |
protectant |
medium |
Do not spray within 30 minutes before dusk to 30 minutes after dawn. Apply fungicide in different FRAC group within days of applying Quintec. |
|
tebuconazole Mettle Gowan |
3 |
Caution |
protectant post-infection anti-sporulant |
medium |
Do not spray on existing PM infections. Spray fresh pruning wounds with 0.5–1.0 oz Mettle per gal. for protection against Eutypa and Botryosphaeria dieback. |
|
myclobutanil Sonoma 2EW Albaugh Inc. |
3 |
Caution |
protectant post-infection anti-sporulant |
medium |
Do not spray on existing PM infections. Spray fresh pruning wounds with 0.2 oz Sonoma 2EW per gal. for protection against Eutypa and Botryosphaeria dieback. |
|
pyraclostrobin + boscalid Pristine BASF |
11,7 |
Caution |
protectant post-infection anti-sporulant |
high |
Do not apply to Concord or Noiret. |
|
benzovindiflupyr Aprovia Syngenta |
7 |
Danger |
protectant post-infection anti-sporulant |
high |
Do not apply more than 0.204 lb benzovindilflupyr/acre/year. |
|
benzovindiflupyr + difenoconazole Aprovia TOP Syngenta |
7, 3 |
Warning |
protectant post-infection anti-sporulant |
high |
Do not apply more than 0.204 lb benzovindilflupyr or more than 0.46 lb difenoconazole/acre/year. May burn non-vinifera varieties. |
|
flutianil Gatten Nichino |
U 13 |
Warning |
protectant post-infection anti-sporulant |
? |
Maximum of 0.16 lb flutianil/acre/year. |
|
fluopyram + tebuconazole Luna Experience Bayer Crop Science |
7, 3 |
Caution |
protectant post-infection anti-sporulant |
high |
Do not apply more than 0.446 lb fluopyram or more than 0.9 lb tebuconazole/acre/year. |
|
isofetamid Kenja 400SC Summit Agro |
7 |
Caution |
protectant post-infection anti-sporulant |
high |
Do not apply a third application of Kenja within 28 days of the second application. Do not apply more than 1.72 lb isofetamid/acre/year. |
|
pydiflumetafen +fludioxinil Miravis Prime Syngenta |
7, 12 |
Caution |
protectant post-infection anti-sporulant |
high |
Do not apply more than 0.36 lb of pydiflumetafen or more than 0.9 lb fludioxinil/acre/year. |
|
pyriofenone Prolivo 300SC Summit Agro |
50 |
Caution |
protectant post-infection anti-sporulant |
medium |
|
|
cyflufenamid Torino Gowan |
U6 |
Caution |
protectant post-infection anti-sporulant |
medium |
|
|
metrafenone Vivando BASF |
50 |
Caution |
protectant post-infection anti-sporulant |
medium |
Do not mix with horticultural oils and do not apply on existing PM infections. |
|
sulfur various formulations and manufacturers |
Multiple sites of action M2 |
Caution |
protectant post-infection anti-sporulant eradicant |
low |
Do not apply within 14 days of applying oil, if temperatures >90°F within 3 days of spray, or on sulfur-sensitive varieties such as Cynthiana/Norton. |
|
paraffinic oil Organic JMS stylet oil JMS Flower Farms |
NC |
Caution |
protectant post-infection anti-sporulant eradicant |
low |
Do not tank mix with Vivando, sulfur, Captan, copper, or fertilizers. Do not apply when temps >90°F, when leaves are wet, when vines are drought stressed, or within 20 days of a copper spray or less than 3 days between applications. |
|
(A more complete listing and fungicide descriptions can be found at http://ipm.ucanr.edu/PMG/r302902111.html/) |
|||||
References
Austin, C.N., and W.F. Wilcox. 2012. Effects of sunlight exposure on grapevine powdery mildew development. Phytopathology, 102, 857–866. Available at http://dx.doi.org/10.1094/PHYTO-07-11-0205
Giese, G., C. Velasco-Cruz, and M. Leonardelli. 2020. Grapevine phenology: Annual growth and development [Guide H-338]. Las Cruces: New Mexico State University Cooperative Extension Service. Available at https://pubs.nmsu.edu/_h/H338.pdf
Grape (Vitis spp.)-Powdery mildew. 2021. In J.W. Pscheidt and C.M. Ocamb (senior eds.), Pacific Northwest Plant Disease Management Handbook. Corvallis: Oregon State University. Available at https://pnwhandbooks.org/node/2784/print
Lowder, S., and B. Warres. 2021, February 1. Early-season scouting for grape powdery mildew. Good Fruit Grower. Available at https://www.goodfruit.com/early-season-scouting-for-grape-powdery-mildew/
Moyer, M., and G. Grove. 2012. Powdery mildew in eastern Washington commercial grape production: Biology and disease management [EM058E]. Pullman: Washington State University Extension.
Moyer, M., M. Cooper, P. Brannen, W. Mahaffee, M. Lewis Ivey, T. Miles, and C. Oliver. 2019, October 31. Good to know: Why some seasons are worse for powdery mildew. Good Fruit Grower. Available at https://www.goodfruit.com/why-some-seasons-are-worse-for-powdery-mildew/
For Further Reading
H-303: Pruning Grapes to the Four-arm Kniffin System
https://pubs.nmsu.edu/_h/H303/
H-322: Propagation of Grape Vine Cuttings: A Practical Guide
https://pubs.nmsu.edu/_h/H322/
H-338: Grapevine Phenology: Annual Growth and Development
https://pubs.nmsu.edu/_h/H338/
Original authors: Bernd Maier, Extension Viticulture Specialist; and Natalie Goldberg, Extension Plant Pathologist. Subsequently revised by Phillip Lujan, Program Manager, and Natalie Goldberg, Distinguished Extension Specialist Emeritus.
Gill Giese is the Extension Viticulture Specialist and an Assistant Professor at NMSU. He earned his Ph.D. at Virginia Tech, and has worked as a commercial winemaker and viticulture/enology instructor. His applied research and Extension work focuses on variety/rootstock evaluation, mitigation of frost/cold damage, soil issues, insects and nematodes, and trellising options to optimize grape yield and berry composition in New Mexico and the arid Southwest.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu.
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
Revised March 2022 Las Cruces, NM