Circular 710
Jason Turner and Shanna LaCount
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Extension Horse Specialist, Cooperative Extension Animal Sciences and Natural Resources, New Mexico State University; Doctor of Veterinary Medicine and Director of Animal Science, New Mexico Junior College. (Print Friendly PDF)

Horses graze on a fenced corrall. Photo by Josh Bachman. NMSU, 2021.
Introduction
The field of equine parasitology has grown more complex in recent years, especially when one considers the application of deworming strategies based on scientific studies. The once simple recommendation of “deworm horses every two months” has become outdated and is strongly opposed by many experts in the field as it is believed to hasten the development of anthelmintic resistance (AR) in parasites which renders common deworming drugs ineffective. The goal of this guide is to educate horse owners on practical applications from this field of study by making them aware of key concepts that support current recommendations. With this knowledge, owners are strongly encouraged to work with their equine veterinarian to discuss control strategies to implement on their own specific equine operation. As this guide is a summary of key findings, readers that wish to expand their knowledge are encouraged to review the sources cited in the References and Further Reading Section.
Major Internal Parasites of Interest
Strongyles. This group includes both large (Strongylus vulgaris) and small (cyathostomes) strongyles. The large strongyle is considered the most pathogenic nematode parasite of horses, but due to the interval deworming programs implemented in the 1960s, it is rarely found in today’s managed horse herds. Conversely, the small strongyle is not considered to be as pathogenic, but it receives greater attention today as it infects horses of all ages. Furthermore, the concern of anthelmintic resistance in small strongyle populations has greatly increased, possibly because of traditional interval deworming programs. As a means to evade the host’s immune system, cyathostomin larvae can invade the lining of the hindgut of the horse to form a cyst. These encysted larvae may remain there for two years or more before they emerge to develop into adults. No known anthelmintic regimen is 100% effective against encysted cyathostomin larvae, so an infected horse can never be truly free of this parasite. Figure 1 shows various stages of the strongyle life cycle by indicating which stages occur in the environment and those that develop within the horse. More attention will be given to these stages later in the circular when we review the role of the environment in parasite development.
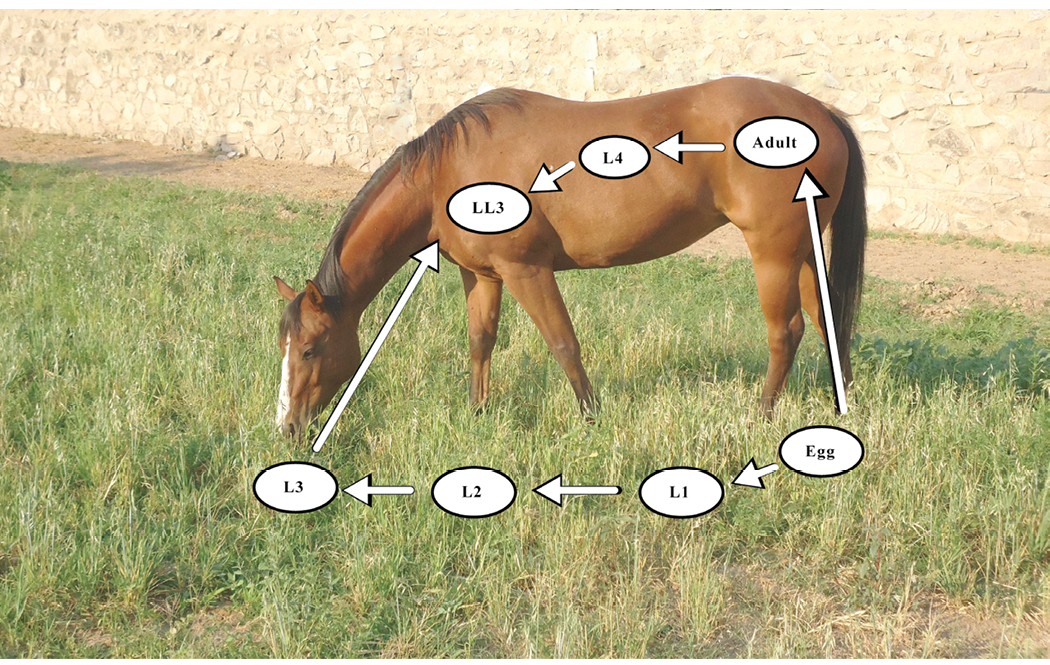
Figure 1. Small strongyle parasite life cycle. Specific developmental stages occur within the horse and on the forage environment. Adult females in the cecum of the horse produce fertilized eggs which are excreted in the feces to contaminate the grazing area. These eggs then hatch into the first-stage larva (L1). Within the feces, the L1 develops into the second-stage larva (L2), and then into the third-stage larva (L3). The L3 migrates from the feces onto the forage where it is ingested by the horse. Within the horse, the L3 develops into the late L3 (LL3) stage, the fourth-stage larva (L4), and then into the fifth stage (L5), or adult, that produces eggs.
Ascarids. The Parascaris spp. group is one of the largest, in terms of physical size, nematodes found in horses. This is a contributing factor in the incidence of impaction colic that can result due to ascarid infection and blockage of the intestine. The adults reside in the small intestine of the horse, and mature adults can often be observed in the feces of foals and other young horses. It is reported that a mature female can produce more than 200,000 eggs per day, and that once larvated, these ascarid eggs in the external environment (see Figure 2 for the Ascarid life cycle) can remain infective for years. Unlike the small strongyle that must develop in a pasture setting before being ingested by the horse, the larvated ascarid egg has a resilient coating that allows it to endure in pasture, dry lot, and stall housing situations where ingestion by the horse occurs. The occurrence of AR in ascarids to common anthelmintic agents has also increased over the past 20 years.
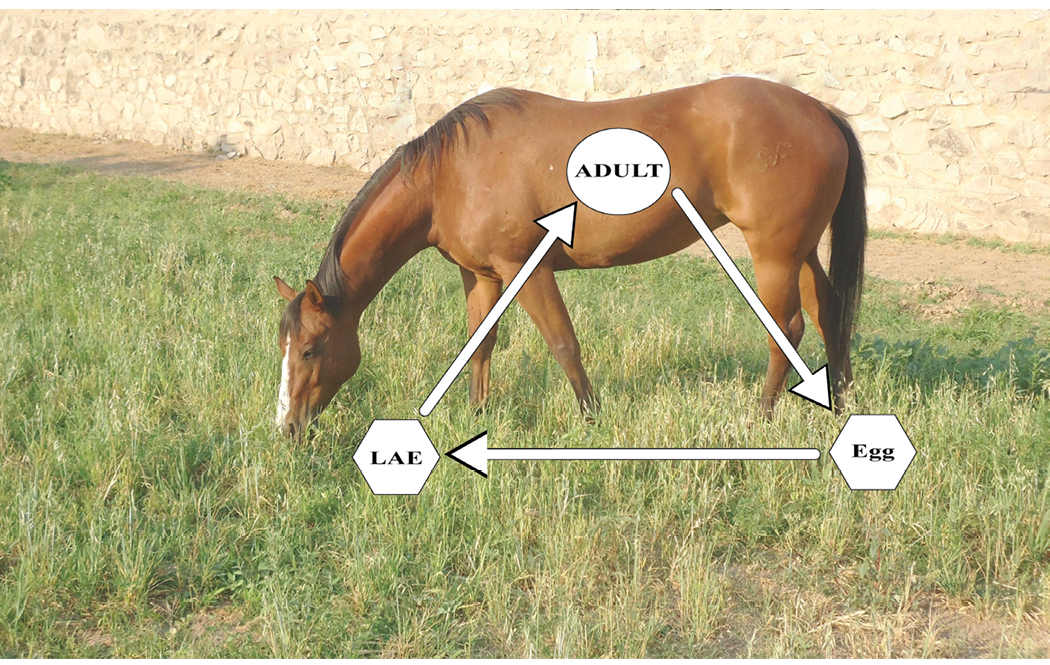
Figure 2. Life cycle of Parascaris species (roundworms). Adult females lay eggs in the small intestine of the horse which are passed in the feces to the environment. The fertilized egg develops within two weeks into a larval stage that can remain infective within the environment for many years. A horse becomes infected by ingesting a larvated ascarid egg (LAE). Within the stomach, the egg loses its protective coating, and the larvae migrate from the gut to the liver and lungs where they further develop before returning to the gut lumen to become egg-laying adults.
Pinworms. The most common pinworm in horses is Oxyuris equi. Pinworms develop in the small intestine and reside in the descending colon and rectum as adults. Adults may be observed in the feces or protruding from the rectum. Females lay eggs on the hairless skin under the tail of the horse around the anus, so eggs are not likely to be observed in a fecal egg count test. While this parasite is not considered a major threat to equine health, the eggs laid under the tailhead can cause itching in horses which leads to intense tail rubbing and, in severe cases, damage to the skin in this area. This tail rubbing can spread pinworm eggs through an equine facility via stall walls, fence posts, grooming materials, tail wraps/bandages/bags, etc. whereby a horse may ingest the larvated eggs. These eggs are hardy and persist in the environment for months. Resistance to certain anthelmintics has also been demonstrated for pinworms.
Bot fly. The life cycle of the bot fly (Gasterophilus spp.) is depicted in Figure 3. Typically, infections within the gut, as the larvae “overwinter” in the digestive tract, do not cause clinical symptoms of disease, so the bot is considered to be less pathogenic than nematode parasites. Since the bot fly only produces one generation per year, it is recommended that owners use an ivermectin or moxidectin product to treat horses for bots in late autumn or early winter.
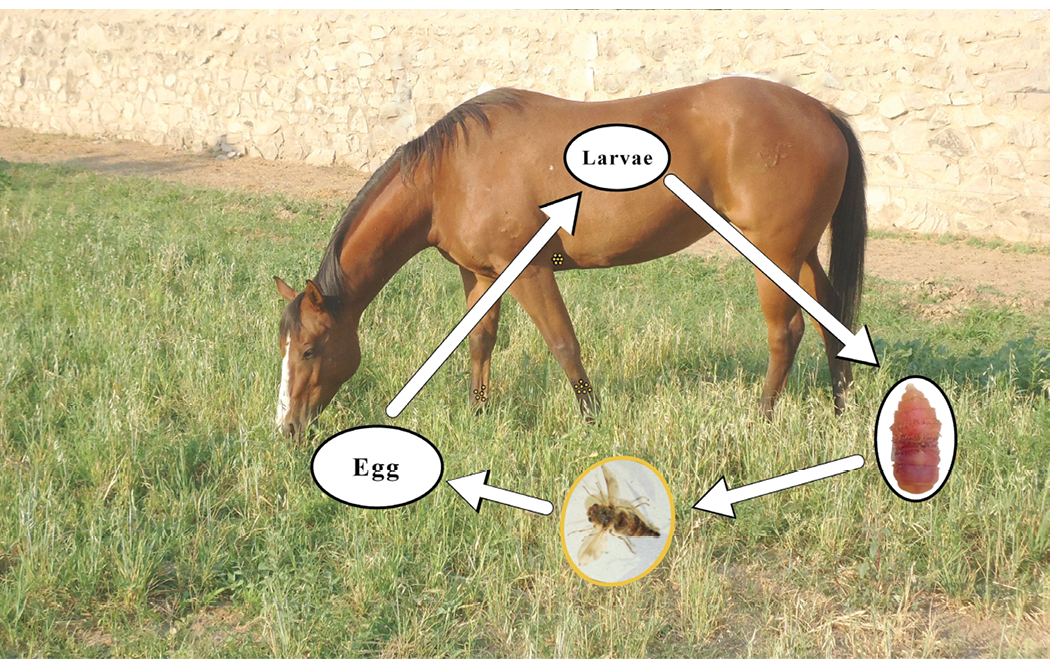
Figure 3. Life cycle of Gasterophilus spp. or bot fly. The female fly deposits eggs on the haircoat of the horse (represented by small yellow dots on the lower legs and behind the elbow). When the horse licks the hair, the eggs enter the digestive tract where they develop into larvae and “overwinter” there. In the spring, these larvae pass from the horse in the feces, where they pupate in the soil to become adult flies during the summer. (13, 14)
Tapeworms. The most common tapeworm in horses is Anoplocephala perfoliata, and the life cycle is illustrated in Figure 4. These parasites typically attach to the lining of the cecum in the horse. Adult worms release body segments that contain eggs into the feces; however, it is rare to observe these segments in the feces unless the horse carries a large tapeworm burden. Fecal egg counting methods also do not accurately detect tapeworm eggs in the feces. Consequently, the recommended test for the presence of tapeworms in a horse is an assay used to detect tapeworm-specific antibodies in the horse’s blood. However, this test reflects exposure to tapeworms rather than the degree of actual infection. The oribatid mite is the required intermediate host for development of tapeworms in the environment. While they are commonly found on grass pastures where they may be ingested by the horse, it is suggested that the prevalence of the mite is lower in arid climates. This suggests that there may be a reduced risk of exposure to tapeworms for horses living in arid regions.
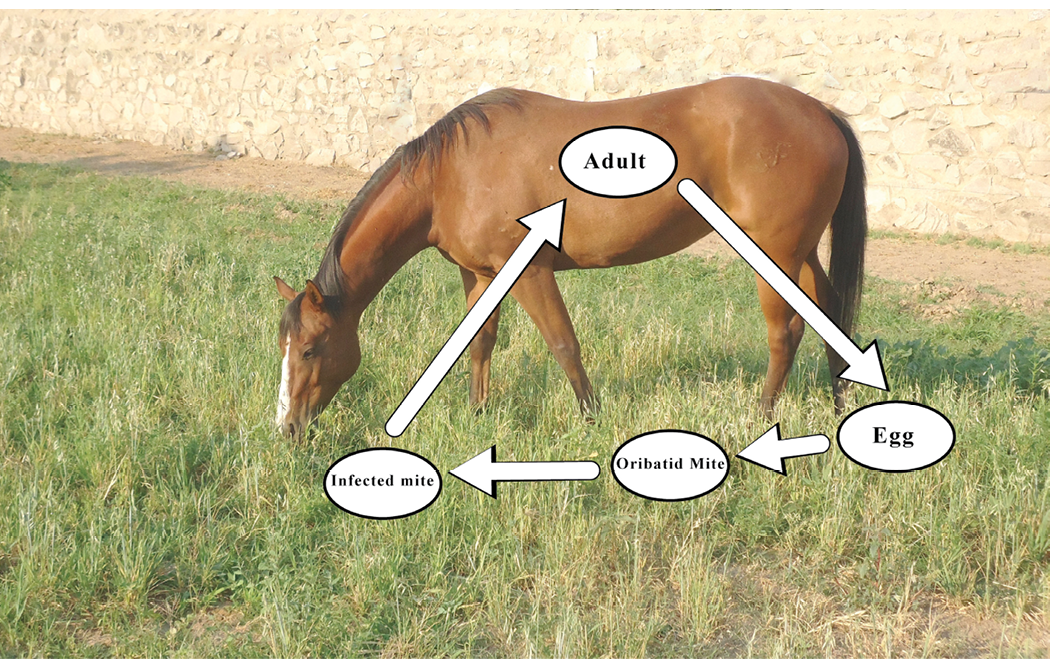
Figure 4. The tapeworm life cycle. Adult tapeworms in the cecum shed body segments that contain eggs which are passed in the feces. Oribatid mites in the environment eat the eggs, and the egg continues to develop into an infective stage. The infected mite is often consumed by the horse along with pasture grass where the tapeworms migrate to the cecum.
History of Deworming Practices for Horses
Prior to the development and use of modern anthelmintic drugs in equine practice, the horse’s own immune system was the primary means of controlling these foreign invaders. Immunity to internal parasites involves both innate and acquired immune responses to deal with the infection. Horses under three years old do not have the same protective level of immunity to some parasites as those acquired by horses aged three and older. For example, most cases of Parascaris spp. infection are observed in horses younger than two years old and are rarer in older horses. For Oxyuris spp., acquired immunity seems to offer protection as the observation of infection is lower in mature horses. In contrast, infection with small strongyles is observed in horses of all ages. However, higher fecal egg counts (FEC) are generally observed in younger horses and those adults that are immunocompromised or under stress. The difference in immunity is believed to be an underlying factor as to why some horses are “low shedders” (less than 200 eggs per gram (EPG) of feces) and some are “high shedders” (greater than 500 EPG of feces) in terms of the FEC. Furthermore, Nielsen suggested that the prepatent period—the time interval between infection of a definitive host with a parasite and the first detection of eggs or larval stages in the feces—for small strongyles may increase in length as horses age and remain exposed to these parasites10. This may in turn have an impact on the suggested interval between deworming treatments for these internal parasites.
To fully understand today’s recommendations for deworming practices, we need to be familiar with the development of anthelmintic agents (deworming drugs) and how their use has impacted equine management today. In the 1940’s, phenothiazine was widely used to control large strongyles, which were the most devastating internal parasite until the 1970’s. During the 1950’s and 1960’s, a “deworming cocktail” given through a nasogastric tube to the horse by a veterinarian was the most common treatment method for parasites. In the 1960’s, new broad spectrum dewormers were introduced, but no standardized deworming schedule existed until a 1966 article in the Journal of the American Veterinary Medical Association suggested deworming horses every six to eight weeks. Paste formulation dewormers became widely available in the 1970’s which allowed owners to commonly administer dewormers to horses without the aid of a veterinarian. Ivermectin was introduced to the market in the 1980’s, and it was innovative as it killed larval forms of internal parasites as well as adults. Moxidectin was introduced in the 1990’s. By the 2000’s, the presence of large strongyles was rare in managed horse herds and resistance to many anthelmintic drugs was reported for the other common internal parasites. This led to the promotion of rotational deworming programs11.
In the winter of 2009-2010, a national survey of over 11,000 horse owners reported that less than 16% of them involved their veterinarian in the deworming of horses, and around 89% reported that ivermectin was the most commonly used dewormer1. In 2015, the National Animal Health Monitoring System of the USDA conducted a survey of over 1900 equine operations, where 78% reported that ivermectin was the most commonly used dewormer. This study reported that only about 8% of operations conducted a FEC test to base deworming practices on, and only about 4% had ever used a FEC test to detect AR7. Prior to the COVID-19 pandemic, a single dose tube of generic ivermectin dewormer for horses could routinely be purchased for less than $5, and sale prices could be as low as $1.99. With the cost of FEC testing being significantly greater, many horse owners may have opted for the cheaper solution without concern for anthelmintic resistance. In 2019, the American Association of Equine Practitioners (AAEP) issued the Internal Parasite Control Guidelines where it recommended more judicious use of dewormers based on FEC test results as a means of minimizing the development of AR in internal parasites3. A subsequent survey suggests horse owners have begun to change their old habits (i.e., deworm every two months) relative to deworming practices. The 2021 American Horse Publications Equine Industry Survey2 of over 7,200 equine owners reported the following positive changes relative to equine deworming practices:
- Around 54% reported that their veterinarian is involved in developing their horses’ deworming program.
- 60% stated that their veterinarian had recommended having a FEC test performed.
- The number of horse owners deworming 1 to 3 times per year had increased, while the number of owners deworming up to 6 times per year had decreased.
Anthelmintic Resistance in Internal Parasites
As mentioned previously, many equine parasitologists believe the two-month interval-dose program introduced in the 1960’s has contributed to the increased incidence of AR to common equine deworming agents observed across the world12. Simply stated, AR occurs when parasites survive a treatment with a deworming chemical that was previously observed to be effective in killing them. As mentioned earlier, the repeated use of the three primary equine anthelmintic drugs (benzimidazoles, pyrimidines, and macrocyclic lactones) can contribute to an increase in the prevalence of internal parasites that develop resistance to these chemicals.
The term refugia refers to the portion of a parasite population that is not exposed to an anthelmintic treatment. This primarily includes free-living parasites present in the environment. Other sources of refugia include parasites in untreated horses or certain parasitic stages (such as encysted small strongyles) that do not come into contact with the deworming drug. In theory, a larger refugia present in the environment reduces the selection pressure on parasites, which would decrease the prevalence of resistant genes in the entire worm population. This helps maintain the effectiveness of deworming drugs or slows the development of resistance. This concept underlies many of the recommendations made by experts today as the presence of a larger refugia should slow the development of AR. This is because resistant parasites are a smaller portion of the entire population and have a less frequent chance of transmitting their resistant genes to subsequent generations, which can increase the incidence of parasites in the environment with widespread resistance to these chemicals.
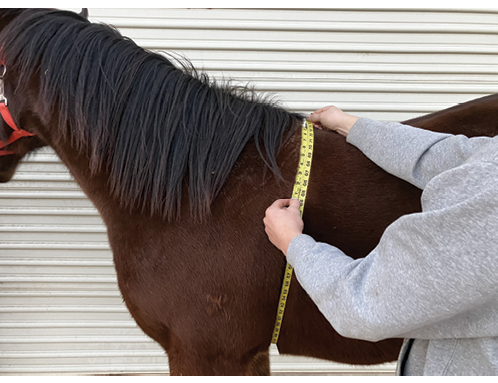
Figure 5. Photo demonstrating how to use a soft tape measure to determine heart girth circumference.
Administering less than the dose recommended on the manufacturer’s label may also contribute to AR in the parasite population. For this reason, owners are encouraged to accurately weigh each horse using a large animal scale or estimate its weight through body measurements. This ensures the proper dose of treatment is determined for each horse. Figure 5 demonstrates using a soft measuring tape to measure the heart girth circumference of a horse. This measurement, along with the body length measurement shown in Figure 6 can be entered in the formula to estimate a horse’s body weight. An insufficient dose can also occur when a horse avoids the paste administration or spits the paste out once it is administered. Therefore, it is important to train horses ahead of time so that they will accept and consume the paste willingly. This can be done using a clean, previously used paste deworming syringe filled with a palatable paste, such as molasses or corn syrup.
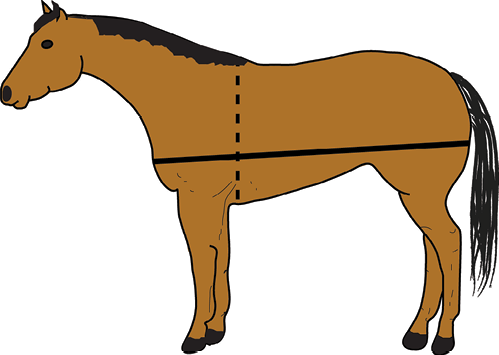
Figure 6. Using a soft tape measure, you can estimate the body weight (in pounds) of the horse by measuring (in inches) the heart girth (dashed line) and the body length (solid line). The formula to estimate is Body weight = (heart girth x heart girth x body length)/330.
Diagnostic Tests
The FEC is the current best test to determine which parasites infect horses and results are normally expressed as EPG of feces. The FEC is used primarily for three purposes: (1) to diagnose clinical infection with internal parasites, (2) as a surveillance method to detect horses that shed a large number of eggs in their feces (“high shedders”), and (3) to calculate the value of the FEC reduction test (FECRT) used to assess the degree of AR. However, this test is not without its shortcomings and the results should be interpreted with caution. Specific limitations include:
- The FEC is best suited for detecting strongyle and ascarid eggs and not eggs from other species.
- The FEC has no direct correlation with the size of the worm burden in the host. It only detects eggs and not the larval stages of parasites which usually have the most pathogenic consequences in the host. While a positive FEC indicates the presence of adult worms in the host, a negative FEC does not ensure the absence of these adults.
- The FEC and FECRT are used to determine AR at the farm level; however, test results from groups with fewer than five horses should be interpreted with caution, unless very high efficacy (greater than 98%) or very low efficacy (less than 80%) for the drug is observed consistently in all the horses tested. It is interesting to note that the FEC for the same horse can vary by 50% even on the same day of testing.
There are a variety of laboratory methods that can be used to perform the FEC which differ in their protocol, sensitivity, accuracy, and precision. Additionally, the threshold value and other variables in the calculation of the FECRT have not been standardized in the past. This makes it difficult to compare results among separate FECRT, which does not allow inferences, with any degree of certainty, to be made beyond the sample of the specific group of horses tested (i.e., AR for ascarids to Drug X on Farm A does not imply there will be AR to Drug X in ascarids on Farm B). This prevents the widespread application of research study results to individual farm management plans. However, the World Association for the Advancement of Veterinary Parasitology has recently published guidelines6 with recommendations for standardizing FECRT protocols.
When used for surveillance purposes of small strongyles, the FEC value has been used to categorize horses based upon the level of environmental contamination shed by that specific animal. It has been estimated that 20% of the horses in a herd are responsible for 80% of the strongyle eggs shed into the environment12. The 2019 AAEP guidelines3 suggest the following categories based upon FEC results:
- Low shedders have less than 200 EPG.
- Moderate shedders are in the range of 200 to 500 EPG.
- High shedders excrete more than 500 EPG of feces in an FEC test.
In selective treatment strategies that attempt to minimize AR, the AAEP guidelines recommend that all adult horses receive one to two dewormings per year, with any additional treatments targeted only to those adults in the high shedder category based on FEC.
A simple formula for the FECRT (AAEP 2019) is: FECRT = ((EPG pre-treatment) – (EPG 14-day post-treatment) / EPG pre-treatment) x 100
The calculated FECRT value is then compared to pre-selected cutoff values to indicate resistance. Generally, these values range from 80-95% depending on which specific anthelmintic is used and a value below that cutoff would indicate some degree of AR in the parasites6.
Aside from the FEC/FECRT, there are other diagnostic tests for the detection of pinworms and tapeworms, and these are summarized in Table 1. This table also provides a summary of AR that has been observed for the three primary classes of equine deworming drugs.
|
Table 1. Summary of Equine Parasites |
|||||||
|---|---|---|---|---|---|---|---|
|
Parasite |
Recommended diagnostic test |
Prepatent Period |
Prevalence |
Pathogenicity |
Impact on host |
Immunity |
Response to Anthelmintics* |
|
Small Strongyles |
Fecal egg count |
2 to 3 months |
Widespread |
Low |
Mass larval emergence from cysts in the hindgut can cause disease. |
Immunity may mitigate disease and egg shedding, but does not prevent infection. |
MLAR PAR BAR |
|
Large Strongyles |
Fecal egg count |
6 to 7 months |
Rare |
Severe |
Signs vary from weight loss to fatal colic. |
Immunity may mitigate disease but does not prevent infection |
ML recommended for control. |
|
Ascarids |
Fecal egg count |
2.5 to 3 months |
Common; mostly in young horses |
Varies from low to severe depending on worm burden |
Weight loss, growth inhibition, diarrhea, intestinal obstruction, colic |
Immunity controls infection |
MLAR PAR B recommended for control. |
|
Pinworms |
Perineal skin scraping for eggs |
Up to 5 months |
Common; mostly in young horses |
Low |
Itchy skin around anus |
Adult horses seem to develop immunity |
MLAR B recommended for control. |
|
Threadworms (Strongyloides westeri) |
Fecal egg count |
Days to weeks |
Low; mostly found in foals less than 6 months old |
Moderate |
Diarrhea |
Immunity develops with age |
Ivermectin and oxibendazole are effective treatments |
|
Bots |
Larva observed in feces |
8 to 10 months |
Widespread |
Low |
Eggs on haircoat may be a nuisance |
Recurrent annual infection |
ML commonly used for control |
|
Tapeworms |
ELISA test for antibody |
1.5 to 4 months |
Common in pastured horses |
Varies from low to severe depending on worm burden |
Serious cases may cause colic complications |
Some horses may develop immunity. |
Q provides effective control |
* Codes: B= Benzimadizoles, such as fenbendazole/oxibendazole; P = Pyrimidines, such as pyrantel pamoate and pyrantel tartrate; ML = Macrocyclic lactones, such as ivermectin and moxidectin; Q = Praziquantel; AR = resistance to this drug has been reported in the USA for the parasite in this row. (American Association of Equine Practitioners, 20193; European Scientific Counsel Companion Animal Parasites, 20194; Merck, 20235; Nielsen, 202210; Reinemeyer & Nielsen, 201312).
Environment and Parasite Development
Climate. Since many internal parasites of horses have life cycle stages that occur in the environment external to the horse’s body (Figures 1 to 4), conditions in this environment can significantly impact the progression of the parasite to an infective stage. Environmental factors that influence parasite development include temperature and moisture. Fortunately, New Mexico’s arid or semi-arid climate, characterized by low precipitation, low humidity, and significant temperature fluctuations, helps control parasite growth. Readers are encouraged to visit the New Mexico Climate Center at https://weather.nmsu.edu/climate/about to learn more about our climate.
A common misconception is that a “killing frost” will kill many parasite stages. While some stages are susceptible, Table 2 shows how specific temperature ranges influence the developmental stages of free-living strongyles. Readers should note that eggs and L3 stages can survive freezing temperatures for months; however, repeated freeze-thaw cycles do not favor survival of fertilized eggs or larva. Conversely, extremely hot temperatures prohibit development and survival of free-living stages. With respect to true ambient temperature, one should consider the parasite’s “microenvironment.” An intact fecal ball can provide insulation against these temperature extremes. Therefore, ambient temperatures may need to be well below 32oF before freezing occurs, or well above 104oF before that temperature is reached within an intact fecal ball.
|
Table 2. Effects of ambient temperature on the free-living stages of strongyles. |
||
|---|---|---|
|
Development |
Temperature Range (oF) |
Survival |
|
No development occurs above this level |
> 104 * |
Free-living stages die rapidly. L3 stage may survive for some weeks inside intact fecal balls. |
|
Optimal range for egg and larvae (L) development to reach L3 stage (inefective) in as few as 4 days. |
77 to 91 *** |
Larvae may survive for a few weeks, but temperature is too high for long-term survival. |
|
Eggs develop into L3 within 2 to 3 weeks. |
50 to 77 ** |
L3 can survive for a few months. |
|
Development takes several weeks to a few months. |
43 to 50 ** |
L3 can survive for many weeks and months. |
|
At temperatures below 43, eggs don’t hatch, and development doesn’t progress. |
32 to 43 ** |
At temperatures slightly above 32, eggs and L3 can survive for several months. |
|
< 32 * |
Non-embryonated eggs and L3 can survive for several months; developing larvae (L1, L2) die. |
|
|
+/- 32 * |
Repeated freeze-thaw events diminish egg and larval survival. |
|
*Temperatures that inhibit development and/or survival. ** Temperatures that favor parasite existence. *** Range that favors development, but not survival.
Modified from: AAEP Internal Parasite Control Guidelines, 20193.
In our arid region, precipitation is seasonal and relative humidity is much lower than other areas of the United States. The typical “Monsoon Season” for New Mexico begins June 15 and lasts through September 30 with 30-40% of our total annual precipitation occurring in July and August. In the “fecal ball microenvironment,” 15-20% moisture in the feces appears to be the lower threshold for larval development to proceed.
Although more study on how climate variables influence parasite development is warranted, a recent study by Nielsen et al. (2019) used computer modeling to evaluate the impact of climate on the development of AR in small strongyles. As there were many variables included in the computer model, readers are encouraged to evaluate the research for themselves. This study8 included climate data for a hot/cold semi-arid climate with low precipitation (Pecos, Texas and a humid subtropical climate with high precipitation (Saint Leo, Florida). Monthly averages for precipitation (Figure 7), low temperature (Figure 8), and high temperature (Figure 9) for Socorro, New Mexico, are provided for comparison to the locations specifically included in the computer model used by Nielsen et al. (2019). Collectively, the climate data for Socorro, New Mexico and Pecos, Texas, are similar, and perhaps the model results would be similar. This study reported that the time (number of model years) before AR was observed was delayed when horses were dewormed two times per year (June and December) when compared to deworming two times per year (March and September), four times per year, or six times per year. Again, this is only from a computer model simulation, but in the absence of site-specific studies in New Mexico, this report suggests that using our climate’s attributes (low moisture and temperature extremes) that hinder parasite development can be beneficial in parasite control as well as delaying the development of AR.
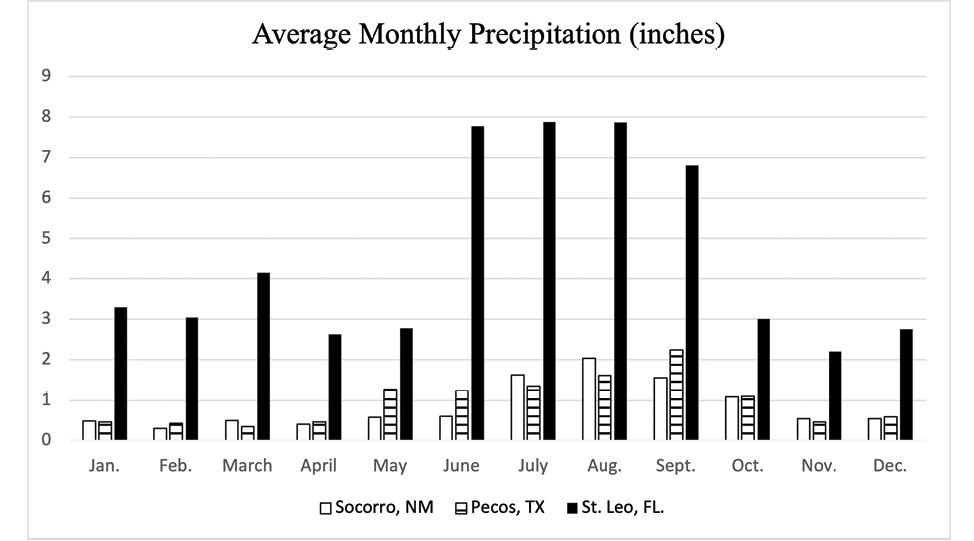
Figure 7. Average monthly precipitation for Socorro, NM, Pecos, TX, and St. Leo, FL. Source: https://www.usclimatedata.com/
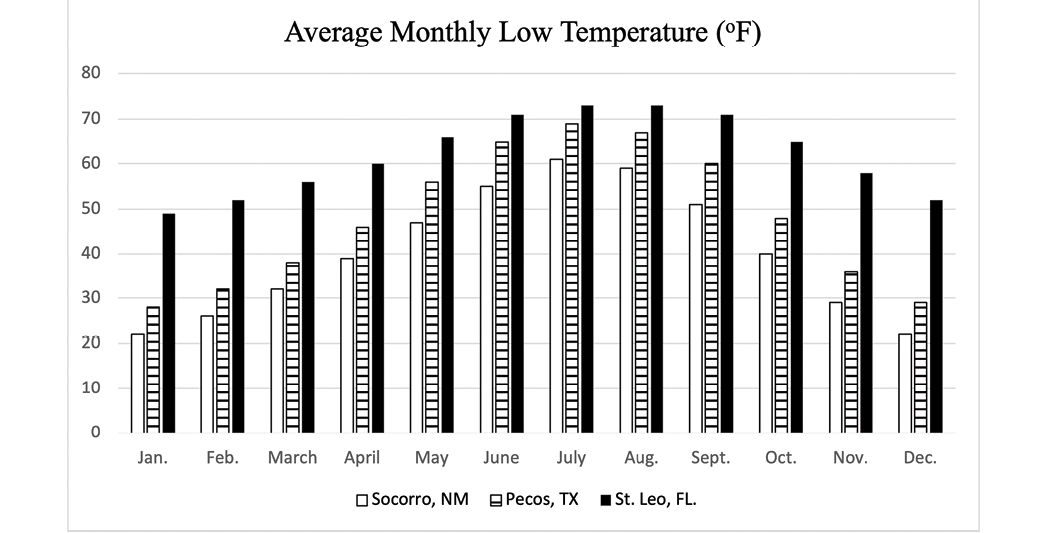
Figure 8. Average monthly low temperature for Socorro, NM, Pecos, TX, and St. Leo, FL. Source: https://www.usclimatedata.com/
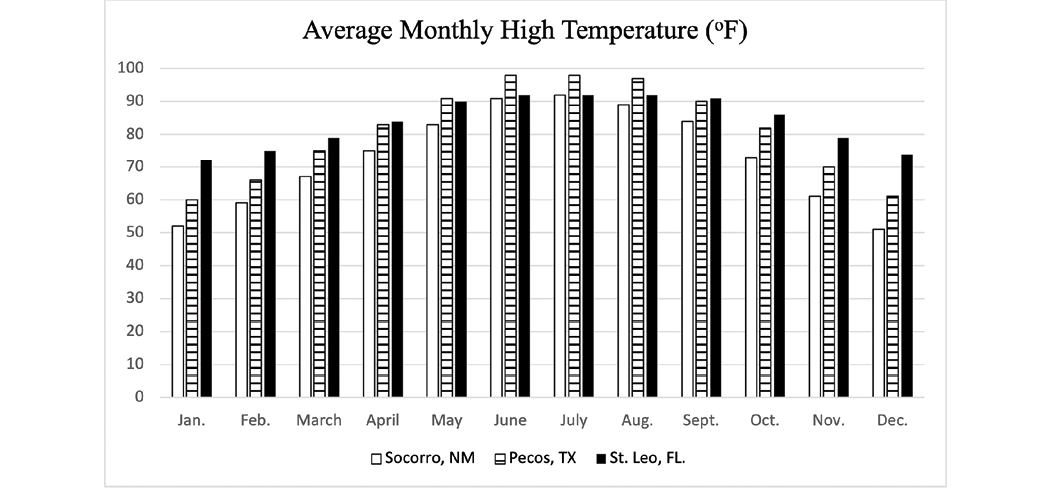
Figure 9. Average monthly high temperature for Socorro, NM, Pecos, TX, and St. Leo, FL. Source: https://www.usclimatedata.com/
Housing. While hardy ascarid and pinworm eggs may develop and be transmitted in non-pasture environments (stalls, barns, dry-lots, etc.), the strongyle develops into an infective L3 larvae and migrates onto pasture vegetation where it is ingested by the horse. Therefore, for stabled horses, exposure to the infective L3 stage of strongyles is lower, and they typically have lower FEC for strongyles than horses maintained on pastures. In this situation, selection of a deworming agent with greater effectiveness against ascarids and pinworms may be warranted. Since ascarid and pinworm eggs are known to persist better in the environment than strongyles, management practices such as composting or drag-harrowing pastures to break up manure piles may aid in control of these parasites. The use of these practices is discussed in greater detail in Guide B-722: “Management Practices for Horse Farms that Aid in Control of Equine Parasites” (2024).
Quarantine. Owners are familiar with the concept of quarantine as the practice of isolating a newly arrived horse on a farm from the rest of the resident herd for a specific period of time to ensure that the new horse does not transmit disease to the rest of the herd. This concept can be applied to deworming as well. Nielsen et al. (2020) reported that macrocyclic lactone-resistant cyathostomins were brought to the USA from Ireland through imported Thoroughbred yearlings9. In this instance, quarantine of these individuals and deworming with an effective anthelmintic drug, with subsequent composting or sanitary disposal of feces containing the parasite eggs, might reduce the incidence of anthelmintic resistance by minimizing the introduction of “resistant genes” from resistant parasites that are allowed to mate with the resident population of parasites present on a farm.
The concept of quarantine can be applied to infectivity as it relates to the pastures that horses graze. Infectivity represents the risk of infection, and it refers to the availability of infective stages of the parasite in the environment. Of the essential equine anthelmintic chemicals, pyrimidines and macrocyclic lactones target larval and adult stages with no ovicidal activity shown against parasite eggs. However, benzimidazoles show both larvicidal and ovicidal modes of action. Most anthelmintics are delivered as a “purge” deworming where a horse receives one dose, or perhaps repeated daily doses for up to five days, to “purge” the horse’s system of the worm burden present at that time. Therefore, great numbers of the resident parasites are excreted in the feces in the few days following a purge deworming. The feces infected with these parasites can be spread across the pasture environment if a horse is immediately returned to the pasture following administration of the dewormer which will likely increase the infectivity of the pasture. Alternatively, dewormed horses could be housed in a dry lot or stall setting for a few days following deworming where the feces could be collected, and composted or otherwise disposed of, without spreading on the pasture to promote infectivity there. Simply put, fewer eggs placed on the pasture means fewer infective larva that can be ingested by the horse grazing the pasture.
Summary Recommendations for New Mexico
The AAEP (2019) identifies the following three priorities as the goal of any parasite control program: (1) to minimize the risk of parasitic disease, (2) to control parasite egg shedding, and (3) to maintain efficacious drugs and avoid further development of anthelmintic resistance as much as possible. A modern deworming program in line with the AAEP priorities requires forethought and judicious use of the anthelmintic drugs currently available. The following suggestions serve as a starting point for equine owners to discuss deworming protocols with their veterinarian, and horse owners are encouraged to seek the advice of a qualified veterinarian before proceeding with any diagnosis, treatment, or therapy.
Horses less than three years of age. These horses are still developing immunity to parasites, and they are generally regarded as “high shedders.” The AAEP recommends that foals be dewormed at least four times during their first year of life. At 2 to 3 months of age, a benzimidazole drug is recommended to target ascarid infections. At weaning (4 to 6 months of age), a FEC is recommended to determine if strongyles or ascarids are the major worm burden with an appropriate dewormer administered to target the most abundant parasites. The third (9 months of age) and fourth (12 months of age) treatments should use drugs targeted toward strongyle control, and inclusion of a product containing praziquantel may be warranted where tapeworm infection is a concern. For the second year of life, three to four treatments are recommended using drugs effective against the primary parasites found in the FEC.
Horses three years of age and over. For these horses that have acquired immunity to some parasites, a two-dose per year program may be sufficient for parasite control. This regimen can also make use of our climatic conditions to optimize the three priorities of the AAEP goal. Deworming around Memorial Day with an anthelmintic effective against the primary worm burden could be used to clear many parasites before horses are placed on summer pastures. Administration of an ivermectin or moxidectin product with praziquantel around Christmas time has the added benefit of controlling tapeworms and bots.
Conclusion
As reviewed in this publication, there is much more that goes into a successful deworming program for horses than to simply “deworm horses every two months.” Owners are encouraged to use the information presented, along with advice from their equine veterinarian, to use all the factors mentioned to develop a multi-faceted and sound approach to deworming horses under their care.
References and Further Reading
- American Horse Publications. (2009-2010). Equine Industry Survey. Retrieved November 11, 2023, from: https://www.americanhorsepubs.org/category/equine-survey/
- American Horse Publications. (2021). Equine Industry Survey Sponsored by Zoetis. Retrieved November 11, 2023, from: https://www.americanhorsepubs.org/2021-equine-survey/
- American Association of Equine Practitioners (AAEP). (2019). Internal Parasite Control Guidelines. Retrieved November 11, 2023, from: https://aaep.org/document/internal-parasite-control-guidelines
- European Scientific Counsel Companion Animal Parasites (ESCCAP). (2019). A Guide to the Treatment and Control of Equine Gastrointestinal Parasite Infections. Retrieved November 11, 2023, from: https://www.esccap.org/guidelines/gl8/
- Gastrointestinal Parasites of Horses. (2023). Merck Veterinary Manual. Retrieved November 11, 2023, from: https://www.merckvetmanual.com/digestive-system/gastrointestinal-parasites-of-horses
- Kaplan, R.M., Denwood, M.J., Nielsen, M.K., Thamsborg, S.M., Torgerson, P.R., Gilleard, J.S., Dobson, R.J., Vercruysse, J., & Levecke, B. (2023). World Association for the Advancement of Veterinary Parasitology (WAAVP) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Veterinary Parasitology, 318. https://doi.org/10.1016/j.vetpar.2023.109936
- National Animal Health Monitoring System. (2015). Equine Management and Select Equine Health Conditions in the United States. United States Department of Agriculture. Retrieved November 11, 2023, from https://www.aphis.usda.gov/animal_health/nahms/equine/downloads/equine15/Eq2015_Rept3.pdf
- Nielsen, M.K., Sauermann, C.W., & Leathwick, D.M. (2019). The effect of climate, season, and treatment intensity on anthelmintic resistance in cyathostomins: A modelling exercise. Veterinary Parasitology, 269, 7–12. https://doi.org/10.1016/j.vetpar.2019.04.003
- Nielsen, M.K., Banahan, M., & Kaplan, R.M. (2020). Importation of macrocyclic lactone resistant cyathostomins on a US Thoroughbred farm. International Journal for Parasitology: Drugs and Drug Resistance, 14, 99–104. https://doi.org/10.1016/j.ijpddr.2020.09.004
- Nielsen, M.K. (2022). Anthelmintic resistance in equine nematodes: current status and emerging trends. International Journal for Parasitology: Drugs and Drug Resistance, 20, 76–88. https://doi.org/10.1016/j.ijpddr.2022.10.005
- Parasite Primer: Part 1-12. (2004). The Horse: Your Guide to Equine Health Care. January-December 2004 Monthly Issues.
- Reinemeyer, C.R., & Nielsen, M.K. (2013). Handbook of Equine Parasite Control. Hoboken, NJ: Wiley-Blackwell, John Wiley & Sons Inc.
- Notafly. (2008). Gasterophilus intestinalis adults. Creative Commons Attribution-ShareAlike 3.0 (CC BY-SA 3.0). https://api.eol.org/media/29641077
- Lamiot. (2005). Gasterophilus intestinalis Larve. Creative Commons Attribution-ShareAlike 3.0 (CC BY-SA 3.0). https://api.eol.org/media/29641087

Jason L. Turner is a Professor and Extension Horse Specialist at NMSU. He was active in 4-H and FFA while growing up in Northeastern Oklahoma. His M.S. and Ph.D. studies concentrated on equine reproduction, health, and management. His Extension programs focus on proper care and management of the horse for youth and adults.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu.
Contents of publications may be freely reproduced, with an appropriate citation, for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
Brand names appearing in publications are for product identification purposes only. No endorsement is intended, nor is criticism implied of similar products not mentioned. Persons using such products assume responsibility for their use in accordance with current label directions of the manufacturer.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
October 2024 Las Cruces, NM


