Guide B-722
Jason L. Turner and Brandon Smythe
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Author: Respectively,Extension Horse Specialist, Extension Animal Sciences and Natural Resources, New Mexico State University; and Research Assistant Professor, Center for Animal Health and Food Safety, New Mexico State University. (Print-Friendly PDF)
NMSU, 2024.
Introduction
Strategies to control internal and external parasites in horses are important for their comfort and health. Internal parasites can cause colic and other complications, while flies and mosquitoes are the vectors responsible for transmission of many viral diseases that infect horses. Many equine parasites rely on environments created by equine manure, rotting feed, forage, and hay, as well as standing water for their development. This is why good sanitation practices are the most important methods of controlling parasites; such practices include proper management of manure, soiled bedding, and wasted feedstuffs, as well as minimizing stagnant water available for insects to breed.
Routine manure management
The best recommendation for manure management is to develop a composting system as the high temperatures (130oF+) that are reached in the process will destroy both internal and external parasite larvae that infect horses. Although composting does require more labor and time than simply spreading manure across pastures, it does a better job in destroying parasites while producing a valuable soil amendment. The University of Massachusetts Extension Service has a brief guide3 on composting that will work well for owners with a couple of horses. The Michigan State University Extension Service has more in-depth information on developing a composting structure and plan for larger farms7.
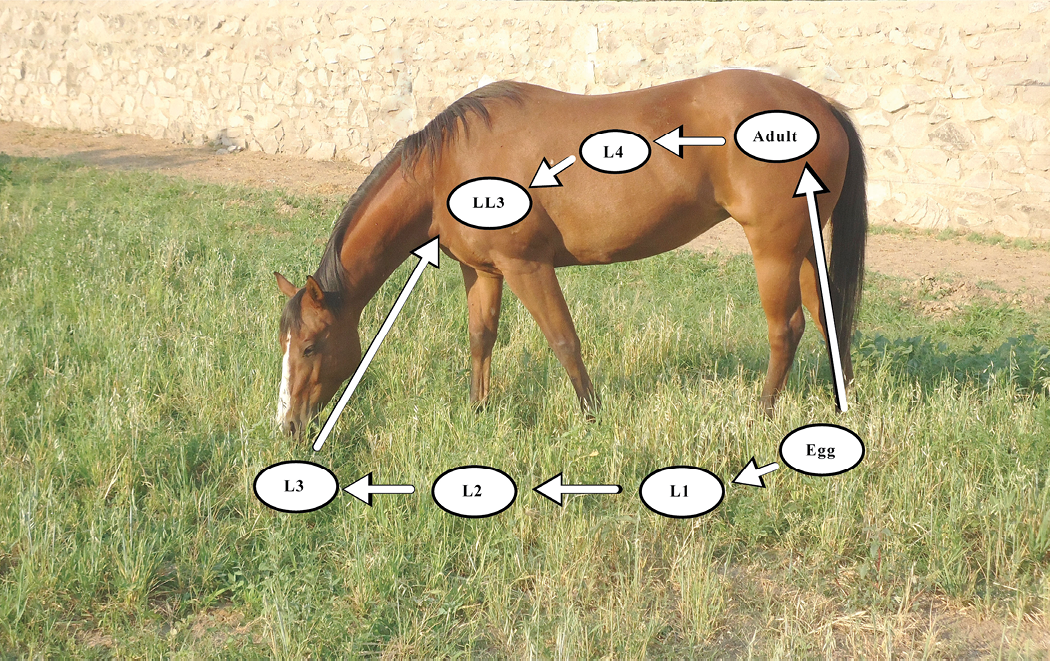
Figure 1. Small strongyle parasite life cycle. Specific developmental stages occur within the horse and on the forage environment. Adult females in the cecum of the horse produce fertilized eggs which are excreted in the feces to contaminate the grazing area. These eggs then hatch into the first-stage larva (L1). Within the feces, the L1 develops into the second-stage larva (L2), and then into the third-stage larva (L3). The L3 migrates from the feces onto the forage where it is ingested by the horse. Within the horse, the L3 develops into the late L3 (LL3) stage, the fourth-stage larva (L4), and then into the fifth stage (L5), or adult, that produces eggs.
Horses shed internal parasite eggs through the feces, and these parasites begin their journey to adulthood from there with various stages of the life cycle occurring in manure and on pastures (Figure 1). Readers are encouraged to review Circular 710 “Internal Parasite Control for Horses in New Mexico” for more detailed information on internal parasites and deworming strategies for horses. Using the small strongyle (known as cyathostomins) as an example, Table 1 shows how ambient temperature impacts the development and survival of different stages of the parasite. Exposing these stages to high temperatures and low moisture levels by physically breaking up the fecal balls by drag-harrowing (Figure 2) pastures can reduce parasite survival. However, this needs to take place during hot summer temperatures and horses should not be grazing pastures when the manure is spread as this will likely increase the chance of infection. The ideal situation would be to keep horses from a pasture where equine manure has been spread for at least one grazing season to allow the equine-specific parasites to die before horses return to grazing there. Alternatively, the pasture could be grazed by a non-equine animal (cattle, sheep, goats, etc.) as the most common equine internal parasites are host-specific6. If horses cannot be removed from grazing the pasture after spreading uncomposted manure, then spreading that manure on pastures used for other livestock, fallow ground, or composting the manure is advised.

Figure 2. A drag harrow being used to break up and spread manure across a dormant pasture.
Many species of flies also rely on moist manure and decaying organic matter (wasted hay and soiled bedding) as breeding grounds. The life cycle of these flies is measured in days. For example, under optimal conditions the house fly can grow from egg to adult in about 10 days. Therefore, it is important that fresh manure be removed, and properly disposed of, from barns and corrals at least every 4 to 5 days during warm, summer months to disrupt fly development. In colder winter months that do not favor fly breeding, the disposal schedule can be less frequent. In instances where manure cannot immediately be added to the compost system or spread over suitable ground (weather delays, etc.), it has been recommended that covering the manure pile with plastic film or a tarp will reduce filth fly development5.
|
Table 1. Effects of ambient temperature on the free-living stages of strongyles. |
||
|---|---|---|
|
Development |
Temperature Range (oF) |
Survival |
|
No development occurs above this level |
> 104 * |
Free-living stages die rapidly. L3 stage may survive for some weeks inside intact fecal balls. |
|
Optimal range for egg and larvae (L) development to reach L3 stage (inefective) in as few as 4 days. |
77 to 91 *** |
Larvae may survive for a few weeks, but temperature is too high for long-term survival. |
|
Eggs develop into L3 within 2 to 3 weeks. |
50 to 77 ** |
L3 can survive for a few months. |
|
Development takes several weeks to a few months. |
43 to 50 ** |
L3 can survive for many weeks and months. |
|
At temperatures below 43, eggs don’t hatch, and development doesn’t progress. |
32 to 43 ** |
At temperatures slightly above 32, eggs and L3 can survive for several months. |
|
< 32 * |
Non-embryonated eggs and L3 can survive for several months; developing larvae (L1, L2) die. |
|
|
+/- 32 * |
Repeated freeze-thaw events diminish egg and larval survival. |
|
*Temperatures that inhibit development and/or survival. ** Temperatures that favor parasite existence. *** Range that favors development, but not survival.
Modified from: AAEP Internal Parasite Control Guidelines2
Pasture Management and Grazing Plans
Good pasture management is a constant challenge on many equine operations, but it is worth the added time and effort. Effective pasture management not only promotes forage health, but it can also have benefits in parasite control. Horses are known to be selective grazers and show “avoidance behavior,” where they avoid grazing in areas in which they defecate when they have a choice. This behavior becomes evident when we look at pastures and can observe “lawns” and “roughs.” The lawns are the closer trimmed areas where they graze higher quality regrowth of the grass, and the roughs are those taller, more mature plants that inhabit the areas where they normally defecate. Since parasites migrate from the fecal balls to complete development, this behavior helps the horse avoid ingesting larger loads of parasites.
Some lines of thought promote mowing pastures to minimize the rough areas and to distribute the grazing more evenly across the pasture. As flies, mosquitoes, and other external parasites may use the rough areas as cover to congregate, there may be times when it is beneficial to mow the roughs for the purpose of minimizing external parasite habitat. However, encouraging grazing in the rough area may increase ingestion of internal parasite larvae. Therefore, mowing rough areas (and drag-harrowing fecal piles) might best be done when horses are removed from a pasture followed by a long period of rest, as previously recommended for drag harrowing or grazing by non-equine livestock. This approach helps reduce the level of internal parasite infection prior to any follow-up grazing by equines.
Proper stocking density, or the number of animals per acre of pasture, is important for maintaining the health, quality, and production of the forage in the lawn areas. Developing internal parasites tend to live in the “thatch” layer near the base of growing plants to take advantage of the higher moisture in this area. If pastures are overstocked, then the lawns are grazed extremely close, bringing horses closer to that thatch layer where they may ingest more parasites. In extreme cases of overstocking, the lawn areas may have a high degree of fecal matter, which further increases the likelihood of ingesting more parasite larvae (Figure 3).

Figure 3. Overstocked pasture where there is little distinction between the lawn and rough areas which increases the likelihood of horses ingesting internal parasites.
Another use of grazing management plans involves grouping horses based on the number of parasite eggs that they shed in their feces. It has been estimated that 20% of the horses in a herd are responsible for 80% of the strongyle eggs shed into the environment6. This can be determined by performing a fecal egg count (FEC) test on each horse, which can be conducted by your veterinarian. The AAEP guidelines2 suggest the following categories based upon FEC results: low shedders have fewer than 200 eggs per gram (EPG) of feces, moderate shedders range from 200 to 500 EPG, and high shedders excrete more than 500 EPG of feces.
In developing a grazing rotation plan, low shedders would graze the cleanest pastures first since they excrete the lowest number of eggs onto a pasture. As the rotation proceeds, the moderate shedders would graze after the low category, and the high shedders would follow the moderate group. Another approach might be to simply group horses into the above categories and have them graze separate pastures knowing that the higher shedding groups will be ingesting more parasite larvae. In the absence of FEC test results for horses, a manager might group horses simply according to age. Generally, horses younger than three years old do not have the level of immunity to internal parasites that is found in horses aged four and over. Consequently, these young horses are likely to be in the high shedder category, and they can be grouped and grazed separately from older horses. The primary goal of this approach is to minimize the ingestion of parasite larvae by those horses that are in the low shedding category, so they do not need to receive deworming as often as those in the higher shedding categories.
Practices for Stabled Horses
For horses maintained in stables, more routine care and inspection allows for management to be implemented at the individual level. It is good practice to regularly inspect the horse and its surroundings for parasites while completing tasks such as grooming and stall cleaning. While grooming, a bot comb or knife can be used to remove bot fly eggs from the haircoat which will minimize ingestion of the larva by the horse. If ticks, mites, lice, or other flies are discovered, then appropriate treatments can be applied. The AAEP External Parasite and Vector Control Guidelines1 is an excellent resource for selecting appropriate treatments for horses suffering from external parasites, and any use of insecticides should follow the directions given on the manufacturer label. While many insecticidal products are available to horse owners for purchase, the effectiveness of these products relies on only a few specific active ingredients that control problematic pests while being safe for animal use. Since insecticidal resistance by pests remains a constant threat, owners should not rely solely on insecticides for complete control or suppression of external parasites. Insecticides complement other control measures as part of a comprehensive Integrated Pest Management program that can help delay the onset of insecticide resistance, minimize problematic pest populations, and insure the health and comfort of horses. As mentioned previously, routine removal, and proper disposal, of manure and wasted feed from stalls at least every other day, if not daily, will help disrupt the life cycle of parasites. If flying insects are a nuisance, then owners should consider fly sheets, masks, and leggings as other options that complement the use of insecticides and repellents on their horses.
Other Complementary Practices
Reduce standing water and wet areas in which mosquitoes or filth flies can develop. This includes draining water from any item that can hold water (e.g., old tires, flowerpots, children’s toys, etc.). Filling low spots or creating a sloping grade around water sources will help minimize standing water in these areas. Tanks or other water sources for animals should be screened and cleaned at least once per week to prevent mosquito growth. When the water source can’t be “cleaned,” use of an approved larvacide, such as Bacillus thuringiensis israelensis can reduce mosquito numbers. To further minimize the risk of exposure, coordinate riding or turn-out times outside of peak mosquito activity, which is normally around dusk and dawn.
The use of parasitoid wasps to control certain filth flies, such as house and stable flies, on horse farms has become popular in recent years. However, the successful suppression of flies with this method has shown mixed results. These wasps deposit eggs into the fly pupa, and the developing wasp kills the host fly as they develop. For a more thorough understanding on the use of parasitoid wasps, the report by Machtinger, Geden, Kaufman, & House5 is recommended reading.
Conclusion
As with any management practice, application of these suggestions will require more thought and labor to carry out than choosing not to implement them. However, these best practices are routinely recommended as the most effective non-chemical means of controlling parasites on the horse farm.
References and Further Reading
- American Association of Equine Practitioners. (2016). External Parasite and Vector Control Guidelines. https://aaep.org/resource/external-parasite-and-vector-control-guidelines/
- American Association of Equine Practitioners. (2019). Internal Parasite Control Guidelines. Retrieved December 8, 2023, from: https://aaep.org/resource/internal-parasite-control-guidelines/
- Herbert, S., Hashemi, M., Chickering-Sears, C., & Weis, S. (n.d.). Composting Horse Manure. University of Massachusetts Extension. https://ag.umass.edu/crops-dairy-livestock-equine/fact-sheets/composting-horse-manure
- Kaufman, P.E., Koehler, P.G., & Butler, J.F. (2019). External Parasites on Horses. Retrieved December 8, 2023, from: https://edis.ifas.ufl.edu/publication/IG139
- Machtinger, E.T., Geden, C.J., Kaufman, P.E., & House, A.M. (2015). Use of Pupal Parasitoids as Biological Control Agents of Filth Flies on Equine Facilities. Retrieved December 8, 2023, from: https://academic.oup.com/jipm/article/6/1/16/2936983
- Reinemeyer, C.R., & Nielsen, M.K. (2013). Handbook of Equine Parasite Control. Hoboken, NJ: Wiley-Blackwell, John Wiley & Sons Inc.
- Skelly, C. (2015). One Horse or a Hundred-What is Composting Anyway? Michigan State University Extension. Retrieved December 8, 2023, from: https://www.canr.msu.edu/resources/one_horse_or_a_hundred_what_is_composting_anyway_wo1022

Jason L. Turner is a Professor and Extension Horse Specialist at NMSU. He was active in 4-H and FFA while growing up in Northeastern Oklahoma. His M.S. and Ph.D. studies concentrated on equine reproduction, health, and management. His Extension programs focus on proper care and management of the horse for youth and adults.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
October 2024 Las Cruces, NM



