Guide A-153
John Idowu, Nicole Pietrasiak, and Mikaela Hoellrich
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Extension Agronomist, Department of Extension Plant Sciences; Assistant Professor, Department of Plant and Environmental Sciences (PES); and Research Assistant, PES, New Mexico State University. (Print Friendly PDF)
Introduction
The soil is home to a variety of microorganisms involved with different processes that enable it to support crop production sustainably. These biological processes affect nutrient cycling and availability, soil aggregation, organic matter decomposition, and crop performance. Some of these important microbially mediated processes are described in this guide: mineralization of the soil organic matter (breakdown of organic materials to release nutrients), immobilization (temporary tying up of nutrients by microbes, thus limiting their availability to plants), nitrogen fixation (conversion of atmospheric nitrogen to forms that crops can use), nitrification (conversion of the ammonium form of nitrogen to nitrate-nitrogen), denitrification (loss of nitrate-nitrogen in the soil through microbial conversion to nitrogen gas), and mycorrhizal associations (the interaction of beneficial soil fungi with plants) (Figure 1).
Figure 1. An illustration describing the interaction of some microbial processes within the soil, plant, and atmosphere.
Mineralization—Breakdown Of Organic Materials To Release Nutrients
Mineralization is the transformation of organic compounds found in organic materials into inorganic compounds. In a nutshell, it means that soil microbes break down organic materials into compounds or ions that plants can use. Many of the elements in organic materials are plant nutrients, but they are locked up in the molecular structure of the substance and are unavailable to plants. Mineralization releases these nutrients into the soil in forms that plants can absorb for growth and development. When organic material is added to the soil, the mineralization process begins, and different types of soil microorganisms begin to break down the organic material. The rate at which soil microorganisms will break down the material depends on environmental factors, including temperature, moisture availability, soil aeration, and the carbon-to-nitrogen ratio (C:N) of the material.
Higher temperatures tend to favor more rapid mineralization compared to lower temperatures (Li et al., 2014) because microorganisms are more active at higher soil temperatures if sufficient moisture is available (MacDonald et al., 1995). Also, chemical reactions can proceed much faster at higher temperatures due to kinetics. Although microbial activities increase with temperature, the optimal fungal and bacterial growth rate occurs at a soil temperature of around 77°F to 86°F (Pietikäinen et al., 2005). When the soil temperature exceeds 86°F, the growth rate of bacteria and fungi decreases rapidly with increasing temperature (Bárcenas-Moreno et al., 2009).
Mineralization also demands adequate soil moisture. If the soil is too dry, the microbes will generally not be active and therefore will not be able to decompose the organic matter adequately. If the soil is too wet, then soil aeration is poor, which is another important factor that reduces mineralization. Just like us, most microbes breathe oxygen. Oxygen-breathing microbes are the most efficient at breaking down organic matter. In poor aeration, microbes don’t have enough oxygen to breathe and therefore will either not survive or will need to find alternative ways to survive—all conditions that slow mineralization and greatly reduce proper decomposition of the soil organic matter. Inadequate oxygen can occur when the soil is saturated with water since the water-filled pores drive the air from the soil.
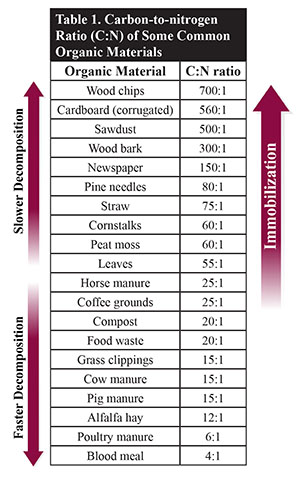
The carbon-to-nitrogen ratio (C:N) is a property inherent in any organic material added to the soil (Table 1). All organic materials contain more carbon than nitrogen atoms in their molecular structure. However, the C:N ratio varies in different organic materials. Materials such as wood shavings and wood chips have a high amount of carbon relative to nitrogen, while materials such as fresh poultry manure and grass clippings have a low C:N ratio. A C:N ratio of 700:1 means the material under consideration (e.g., wood chips; Table 1) has 700 parts of carbon to one part of nitrogen, while a ratio of 4:1 (e.g., blood meal; Table 1) means the material has four parts of carbon to one part of nitrogen. Therefore, there is more nitrogen in a material with a C:N ratio of 4:1 compared to 700:1. Since nitrogen is often more limiting than carbon when organic materials are being broken down, materials with a high C:N ratio will take a longer time to break down due to a scarcity of nitrogen, while materials with a low C:N ratio break down faster due to a relative abundance of nitrogen.
Immobilization—Temporary Tying Up Of Nutrients By Microbes
Immobilization is a process in which soil microorganisms take up nutrients from the soil, especially nitrogen. Microbes need nitrogen to grow and multiply so they can break down more organic material, especially organic matter with a high C:N ratio. Therefore, the nitrogen can be tied up in microbial biomass or can accumulate in byproducts of microbial activity. During the period of immobilization, the soil can undergo nutrient deficiency. However, this process is temporary since the immobilized nutrients can be released back into the soil when microbes die and nutrients within their tissues are recycled.
Materials such as sawdust or wood chips, which have high C:N ratios, can cause immobilization when incorporated into the soil. This material does not contain enough nitrogen for continued rapid microbial decomposition. If sawdust is incorporated into the soil during the crop growing season, the plants can experience nitrogen deficiency unless additional nitrogen is added to the soil. To prevent immobilization, materials with a very high C:N ratio should not be incorporated into the soil. Instead, they can be composted or used as mulches to cover the soil surface to prevent moisture loss, suppress weeds, and reduce soil temperature, especially during hot summer days. The C:N ratios of some common organic materials are presented in Table 1.
Nitrogen Fixation—Conversion Of Atmospheric Nitrogen Gas To Ammonium
Nitrogen fixation is a microbially mediated process that converts nitrogen gas to plant-available forms of nitrogen. Nitrogen is a plant nutrient needed in the highest quantity in most crops. However, crops cannot use nitrogen gas from the atmosphere to meet their nitrogen demand. That is why a substantial amount of nitrogen fertilizer is used by farmers each year. Mineral fertilizers made in the factory, such as ammonium nitrate, contain soluble ammonium and nitrates that plants can directly use for their growth. However, there are bacteria in the soil that can convert nitrogen gas to soluble forms of nitrogen for crop uptake, and this process is called nitrogen fixation.
Two types of nitrogen fixation can happen in the soil. The first type is non-symbiotic nitrogen fixation performed by bacteria called free-living nitrogen fixers. Examples of such bacteria include Azotobacter, which is a Proteobacterium, and Cyanobacteria, such as Nostoc or Scytonema. The estimate of nitrogen fixed by the free-living microbes in arid agricultural lands is relatively low at about 4 lb nitrogen/acre/year (Vlek et al., 1981). However, in arid rangelands and unmanaged lands, nitrogen fixation by soil cyanobacteria can be much higher, especially when they form soil surface aggregates known as biological soil crusts (Figure 2; Elbert et al., 2012).
Figure 2. Cyanobacteria in rangelands and unmanaged lands can form biological soil crusts, which represent living soil aggregates that stabilize the desert soil. Biological soil crusts are home to myriad diverse microorganisms, including cyanobacteria, other bacteria, eukaryotic algae, mosses, and lichens. Shown here are lichen and cyanobacteria crusts.
The other type of nitrogen fixation is symbiotic, and this occurs between the Rhizobium bacteria and legumes. In symbiotic nitrogen fixation, a legume plant forms a mutually beneficial relationship with Rhizobium bacteria. The Rhizobium bacteria are attracted to the cells of the root hairs because the legume plant secretes specific chemicals (flavonoids) into the soil through its roots. Once the bacteria have “infected” the root hairs, the plant cells respond by dividing and creating root nodules, which house the bacteria. The Rhizobium bacteria in the nodules convert nitrogen gas, which is abundant in the soil, into ammonia, which plants can utilize. The legume receives the bacterially fixed nitrogen products, while the bacteria in the nodules receive carbohydrates from the legume, allowing them to thrive. This symbiotic nitrogen fixation can play a significant role in nitrogen management of arid and semiarid soils. Including legume cover crops in rotation can provide significant amounts of nitrogen for subsequent crops after the legume cover crop is terminated and worked back into the soil. Up to 250 lb nitrogen/acre/year can be fixed through the symbiotic association of Rhizobium bacteria with legumes in agricultural lands (Roper and Gupta, 2016). Figure 3 shows an example of nodules attached to the roots of a legume plant.
Figure 3. Nodules on the roots of hairy vetch.
Nitrification—Conversion Of Ammonium To Nitrate-Nitrogen
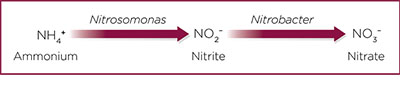
Nitrification is the conversion of ammonia or ammonium ions to nitrate through bacterial action. Nitrification is a two-step process in the soil; first, the ammonia or ammonium compound is converted to nitrite by bacteria in the genus Nitrosomonas, and second, the nitrite is converted to nitrate by bacteria in the genus Nitrobacter (Figure 4).
Figure 4. Nitrification process in the soil.
Since nitrification is an oxidative process (a process requiring oxygen), the soil must be well aerated for nitrification to take place. Nitrification also requires a warm temperature for optimal processing, often greater than 70°F, and thus progresses very slowly at low temperatures. Nitrification is an important process in the soil because it can control how much nitrate is available in the soil. Plants can make use of both nitrate and ammonium forms of nitrogen. Ammonium-nitrogen is a positively charged ion that often gets attached to the small humus or clay particles in the soil in a process called adsorption. When this happens, ammonium-nitrogen may be more difficult for the plants to access, depending on how tightly the ammonium ions are held by the clay particles. However, the ammonium-nitrogen that is not so tightly held by the clay particles will be available for crop uptake. Nitrate, in contrast, is a more readily available form of nitrogen in the soil, and it can be absorbed by the plants faster than ammonium-nitrogen because it stays mostly dissolved in soil water. When too much nitrate is in the soil, it can easily dissolve in soil water and be leached as water moves through the soil. When the nitrate-nitrogen is moved down through the soil beyond the rooting zone, it becomes unavailable for crop uptake, and it can eventually end up in the groundwater. Another problem of excessive nitrate in the soil is the possibility for it to be denitrified, a process where some bacteria can convert nitrates to nitrogen gases under certain conditions—another pathway through which nitrogen is lost from the soil.
Ammonium-nitrogen can be introduced to the soil through manure, composts, decomposing crop residues, and mineral fertilizers used in commercial agriculture. Some commercial farmers use nitrification inhibitors to prevent nitrification from progressing at a rapid rate when they use ammonium fertilizers for the nitrogen nutrition of plants. Since the ammonium-nitrogen can be held by humus and clay particles, it is not easily subject to leaching compared to nitrate-nitrogen. This means that the plants that are growing in the soil will have more time to use the nitrogen in ammonium form before it is converted to nitrate-nitrogen, which can be lost through leaching. Nitrification inhibitors function by affecting the activities of the Nitrosomonas bacteria, preventing them from converting ammonium to nitrites (Figure 4). Since nitrification inhibitors interrupt the pathway of ammonium conversion to nitrate, they will also reduce the amount of nitrate that may be denitrified or leached from the soil. In general, the nitrogen use efficiency of crops can be improved by nitrification inhibitors since they will keep nitrogen longer in the soil in the ammonium form, which prevents it from potential losses and allows plants to use it for growth and development.
Denitrification—Conversion Of Nitrate To Nitrogen Gas
Denitrification is the chemical conversion by microbes of nitrates (NO3-) and nitrites (NO2-) present in the soil to nitric oxide (NO), nitrous oxide (N2O), and nitrogen gases (N2) (Figure 5). Denitrifying bacteria are facultative anaerobes, which means they ordinarily flourish in an oxygen-rich environment, but when oxygen is scarce, they shift their metabolism and start using soil nitrate and nitrites to breathe, in the process known as respiration. This implies that important plant-accessible nitrogen in the soil can be lost through denitrification, lowering the quantity of nitrogen available for crop production. Thiobacillus denitrificans, Micrococcus denitrificans, Pseudomonas, and Serratia species are among the bacteria that can cause denitrification in the soil. Research has shown that, depending on the soil condition, up to 20% of the nitrogen applied to the soil can be lost through denitrification (Colbourn and Dowdell, 1984). Another detrimental effect of denitrification occurs if the end product of the denitrification is nitrous oxide (N2O) because this gas is known to be 300 times more powerful than carbon dioxide in causing a global warming effect. When soil aeration is poor, as it is when the soil is standing in water for a lengthy period, a suitable environment for denitrification exists. Therefore, it is critical not to over-irrigate or compact the soil to the point that it becomes saturated for an extended time. Denitrification and subsequent nitrogen loss from the soil will be favored in such a situation.
Figure 5. Denitrification process in the soil.
Mycorrhizal Association—Beneficial Fungal Association With Plant Roots
Mycorrhizal association occurs in the soil when mycorrhizal fungi (MF) form a symbiotic partnership with plant roots to help the plant with the uptake of water and nutrients, especially phosphorus. In return, the plants provide simple carbohydrates for the growth of the MF. The MF association is very common among most plants, and more than 80% of all land plants can form this association. Based on the way that the MF associate with plants, two major types of association can be defined: ectomycorrhizal and endomycorrhizal fungi.
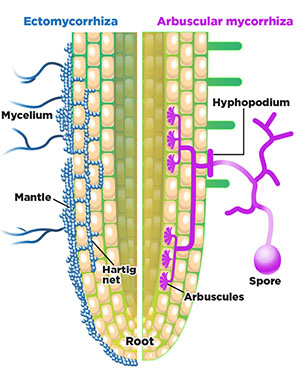
In the first type—ectomycorrhizal association—the MF infect the plant roots and multiply in the cell wall region without penetrating the cell wall. Ectomycorrhizal fungi wrap themselves around the outer portion of the roots and grow outward into the soil (Figures 6 and 7). The ectomycorrhizal association is more prevalent among forest trees.
Figure 6. Ectomycorrhizal fungi (left, blue) and endomycorrhizal (arbuscular) fungi (right, purple) interactions with a plant root (adapted from Bonfante and Genre, 2010). The ectomycorrhiza grow around—but not into—the plant cell walls, while the endomycorrhiza grow into plant cells and form arbuscules.
Figure 7. Mycorrhizal fungi growing around New Mexico pecan roots.
In the second type—endomycorrhizal association—the MF penetrate the cell wall and grow into the plant cells. Endomycorrhizal fungi develop tiny, highly branching structures inside plant cells, called arbuscules, that are used to exchange nutrients and carbohydrates between the plant and the fungi (Figure 6). Endomycorrhizal fungi are often called arbuscular mycorrhizal fungi (AMF). The AMF is of greater importance in farming because most native plants, field crops, and vegetable crops can form a symbiotic relationship with the AMF.
In both types of association, after the MF associate with the plant roots, they keep growing outward, potentially extending the zone from which nutrients and water can be extracted and directed back to plant roots. At the same time, the MF receive simple carbohydrates from the plant to enable the fungi to continue to grow. This relationship is called a symbiotic association. The MF are also called “root extenders,” and due to this root-extending function, the MF association increases the efficiency of plant mineral uptake from the soil and helps plants to be more drought-resilient. Some studies have also shown that the AMF can solubilize nutrient elements like phosphorus and sulfur and make them available for crops. It has also been shown that the AMF can help alleviate other stresses on plants, such as extreme temperature, pH, toxic metals, soil pathogens, and soil salinity (Wu, 2017).
Another key advantage of the AMF is their ability to enhance stable soil structure. They do this directly with their hyphae (vegetative growth structures), forming a physical network around the soil particles, and indirectly by exuding an iron-containing protein called glomalin (Yang et al., 2017). Glomalin is a very sticky substance that acts as a “biological glue” for the soil crumbs. Although many plants can form AMF associations, their occurrence may be limited in croplands because of frequent soil tillage, and it has been shown that the AMF association is more abundant in untilled soils compared to frequently tilled soils (Kabir, 2005).
Another way that plants obtain nutrients from the soil is through a process called the rhizophagy cycle. In the rhizophagy cycle, non-pathogenic soil microbes called endophytes are attracted to plant roots through the exudates secreted by plants into the soil. Some of the endophytes eventually enter the roots and start to live in the plant tissues. Through certain substances in the plant roots called “superoxides,” the cell walls of the microbes are dissolved, and nutrients contained within the microbes are released into the plants (White et al., 2018). After the extraction of nutrients from the microbes, they move back into the soil via the root tips, where they acquire more nutrients before re-entering the plant roots. In this way, these microbes alternate between living inside the plants and living freely in the soil. The rhizophagy cycle is highly beneficial to plants for the acquisition of plant micronutrients (Vishwakarma et al., 2021).
Measuring Soil Biological Processes
Precise measurements of biological processes often require advanced laboratory and field methods. Some of the processes can be measured on disturbed soil samples, while others must be performed on undisturbed soils (in situ) in the field.
Some simple field-based methods can be used to indirectly assess some of these biological processes, such as the tea bag method for estimating the decomposition rate (Keuskamp et al., 2013) and the Solvita field soil test for estimating soil respiration (Doran et al., 1997). These measurements are indicators of total microbial activity in the soil. There are other methods developed by different authors for estimating these biological processes. Whichever method is used, it is important to read the instructions carefully to get reliable results. Several laboratories also offer soil biological measurements, especially for tests that can be performed on disturbed soil samples. Before submitting samples to a laboratory for analysis, it is a good idea to study the sampling protocol required for that measurement.
Conclusion
Soil microorganisms are essential for improving soil health and ensuring its sustainability. They do this by affecting nutrient transformation and availability in the soil through a variety of processes. They also play a crucial role in the transformation of organic materials that are added to the soil. To be able to manage the soil sustainably, it is necessary to understand the roles that microbes perform in the soil and the numerous activities that they carry out. Soils of arid and semiarid regions are particularly challenging to manage, and a major issue is lower biological activities in such soils due to low organic matter contents. Adding organic material to the soil, such as plant residues, composts, or manure, will help stimulate soil biological activities, which will eventually lead to improved soil health.
References
Bárcenas-Moreno, G., M. Gómez-Brandón, J. Rousk, and E. Bååth. 2009. Adaptation of soil microbial communities to temperature: Comparison of fungi and bacteria in a laboratory experiment. Global Change Biology, 15(12), 2950–2957.
Bonfante, P., and A. Genre. 2010. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nature Communications, 1(1), 1–11.
Colbourn, P., and R.J. Dowdell. 1984. Denitrification in field soils. Plant and Soil, 76(1), 213–226.
Doran, J., T. Kettler, and M. Tsivou. 1997. Field and laboratory Solvita soil test evaluation [Manuscript]. Lincoln: University of Nebraska, USDA–ARS.
Elbert, W., B. Weber, S. Burrows, J. Steinkamp, B. Büdel, M.O. Andreae, and U. Pöschl. 2012. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nature Geoscience, 5(7), 459–462.
Kabir, Z. 2005. Tillage or no-tillage: Impact on mycorrhizae. Canadian Journal of Plant Science, 85(1), 23–29.
Keuskamp, J.A., B.J. Dingemans, T. Lehtinen, J.M. Sarneel, and M.M. Hefting. 2013. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods in Ecology and Evolution, 4(11), 1070–1075.
Li, Y., Y. Liu, Y. Wang, L. Niu, X. Xu, and Y. Tian. 2014. Interactive effects of soil temperature and moisture on soil N mineralization in a Stipa krylovii grassland in Inner Mongolia, China. Journal of Arid Land, 6(5), 571–580.
MacDonald, N.W., D.R. Zak, and K.S. Pregitzer. 1995. Temperature effects on kinetics of microbial respiration and net nitrogen and sulfur mineralization. Soil Science Society of America Journal, 59(1), 233–240.
Pietikäinen, J., M. Pettersson, and E. Bååth. 2005. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiology Ecology, 52(1), 49–58.
Roper, M.M., and V.V.S.R. Gupta. 2016. Enhancing non-symbiotic N2 fixation in agriculture. The Open Agriculture Journal, 10(1), 7–27.
Vishwakarma, K., N. Kumar, C. Shandilya, and A. Varma. 2021. Unravelling the role of endophytes in micronutrient uptake and enhanced crop productivity. In N. Shrivastava, S. Mahajan, and A. Varma (Eds.), Symbiotic Soil Microorganisms (pp. 63–85). New York, NY: Springer Cham.
Vlek, P.L.G., I.R.P. Fillery, and J.R. Burford. 1981. Accession, transformation, and loss of nitrogen in soils of the arid region. Plant and Soil, 58(1), 133–175.
White, J.F., K.L. Kingsley, S.K. Verma, and K.P. Kowalski. 2018. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms, 6(3), 95.
Wu, Q.S. (Ed.). 2017. Arbuscular mycorrhizas and stress tolerance of plants. New York, NY: Springer.
Yang, Y., C. He, L. Huang, Y. Ban, and M. Tang. 2017. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLOS ONE, 12(8), p.e0182264.
For Further Reading
A-129: Nitrogen Fixing by Legumes
https://pubs.nmsu.edu/_a/A129/index.html
A-150: Principles of Cover Cropping for Arid and Semi-arid Farming Systems
https://pubs.nmsu.edu/_a/A150/index.html
CR-694B: Soil Health—Importance, Assessment, and Management
https://pubs.nmsu.edu/_circulars/CR694B/index.html
John Idowu is an Extension Agronomist in the Department of Extension Plant Sciences at NMSU. He earned his master’s in agronomy from the University of Göttingen in Germany and his Ph.D. in land management from Cranfield University in the UK. His research and Extension activities are focused on sustainable crop production and soil management in New Mexico.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
January 2023 Las Cruces, NM