Guide A-123
Robert Flynn, Extension Agronomy Specialist Shane T. Ball, Extension Agronomy Specialist R.D.Baker, Extension Agronomist
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University. (Print Friendly PDF)
Of the many factors affecting crop quality and yield, fertility is one of the most important. It is fortunate that producers can control fertility by managing the plant's nutritional status. Nutrient status is an unseen factor in plant growth, except when imbalances become so severe that visual symptoms appear on the plant.
The only way to know whether a crop is adequately nourished is to have the plant tissue analyzed during the growing season.
What Plant Tissue Analysis Shows
Plant tissue analysis shows the nutrient status of plants at the time of sampling. This, in turn, shows whether soil nutrient supplies are adequate. In addition, plant tissue analysis will detect unseen deficiencies and may confirm visual symptoms of deficiencies. Toxic levels also may be detected. Though usually used as a diagnostic tool for future correction of nutrient problems, plant tissue analysis from young plants will allow a corrective fertilizer application that same season.
Not all abnormal appearances are due to a deficiency. Some may be due to too much of certain elements. Also, symptoms of one deficiency may look like those of another. A plant tissue analysis can pinpoint the cause, if it is nutritional. A plant analysis is of little value if the plants come from fields that are infested with weeds, insects, disease organisms; if the plants are stressed for moisture; or if plants have some mechanical injury.
The most important use of plant analysis is as a monitoring tool for determining the adequacy of current fertilization practices. Sampling a crop periodically during the season or once each year provides a record of its nutrient content that can be used through the growing season or from year to year. With soil test information and a plant analysis report, a producer can closely tailor fertilization practices to specific soil-plant needs.
It also may be possible to prevent nutrient stress in a crop if the plant analysis indicates a potential problem developing early in the season. Corrective measures can be applied during the season or, if the crop is perennial, during the next year. Combined with data from a soil analysis, a tissue analysis is an important tool in determining nutrient requirements of a crop. By request, the following elements can be determined in a plant sample:
| Nitrogen | Sulfur | Boron |
| Phosphorus | Iron | Sodium |
| Potassium | Copper | Chlorine |
| Calcium | Zinc | Molybdenum |
| Magnesium | Manganese |
Levels of elements such as cadmium, lead, arsenic, and selenium also can be examined. See table 1 for sufficiency levels of plant nutrients.
Collecting and Preparing the Sample
If you suspect a nutrient deficiency:
- Sample when the symptom first appears (see table 2 for deficiency symptoms).
- In the same field or area, collect similar samples of plant materials from plants that appear abnormal.
- Make sure that the symptoms are not due to a factor unrelated to plant nutrition.
The parts of plants to sample depend on the plant and its growth stage. Table 3 lists the best parts to sample for common crops (see also fig. 1). More specific sampling strategies may be necessary for cotton and peppers (chile). Also, many devices are available for a "quick test" of the plant nitrogen status. Chlorophyll meters for certain crops can be used to predict the cost/benefit of additional nitrogen fertilizer.
Instructions for petiole or leaf sampling may differ. Also, comparing samples from both a "good" and a "bad" area often helps in determining corrective action. If specific sampling guidelines are not given here, collect recently mature leaves just below the growing point from at least 10 plants.
When gathering the tissue sample in the field, use a clean container. A plastic pail or a paper bag works best. Never use a metal container because it can contaminate the sample.
If the plant samples have soil, fertilizer, dust, or spray residues on them, they will need to be cleaned. A dry brush works best, but for stubborn residues, wipe the samples with a damp cloth or wash them with distilled or deionized water. However, do not prolong the washing because it can leach nutrients out of the tissue.
Air-dry the samples in the shade, not in the sun. To prevent contamination, place the dried samples into clean paper bags or envelops for mailing to the laboratory. Never place fresh plant tissue samples in plastic bags for mailing. The plastic bags do not allow the samples to dry, so they may decompose. It is also a good idea to take a soil sample in the same vicinity as the plant sample because the soil test may help to interpret the plant tissue analysis readings. Mail the samples to: Soil, Water, and Air Testing Laboratory / New Mexico State University / Gerald Thomas Hall, room 269 / P.O. Box 30003, MSC 3Q / Las Cruces, NM 88003.
A nominal fee will be charged. Your county Extension agent can provide further details.
Provide Information with the Sample
When mailing samples to the laboratory, be sure to provide the following information:
- Type of crop.
- Variety.
- Soil type (if known).
- Current crop fertilization and management practices (such as stand, kinds and rates of fertilizer, method of fertilizer application).
- Last year's crop fertilization practices and yield.
- Irrigation frequency and quality of irrigation water.
- Visual appearance of crop.
- Insect and disease problems (if any).
This information is necessary for sound interpretation of the plant tissue analysis.
Things to Avoid
Do not sample the following:
- Young, emerging leaves; old, mature leaves; and seeds. These plant parts usually are not suitable because they are not likely to reflect the nutrient status of the whole plant.
- Diseased or dead plants.
- Plants that have insect or mechanical damage.
A single plant showing visual deficiency symptoms, unless it is possible to sample normal plants from an adjacent area in the field. Normal plants give a reference to help interpret the chemical analysis of the deficient plant sample.
| Table 1. Sufficiency levels of plant nutrients for crops at growth stages shown in table 3.* | |||||||
| Sufficiency levels | |||||||
| Element | Corn | Grain sorghum | Soybeans | Small grains | Peanuts | Alfalfa | Bermuda grass |
| Nitrogen, % | 2.7-3.5 | 3.3-4.0 | 4.2-5.5 | 1.7-3.0 | 3.5-4.5 | 4.5-5.0 | 2.5-3.0 |
| Phosphorus, % | .25-.40 | .20-.35 | .26-.50 | .20-.50 | .20-.35 | .26-.70 | .26-.32 |
| Potassium, % | 1.7-2.5 | 1.4-2.5 | 1.7-2.5 | 1.5-3.0 | 1.7-3.0 | 2.0-3.5 | 1.8-2.1 |
| Calcium, % | .21-1.0 | .30-.60 | .36-2.0 | .20-.50 | 1.25-1.75 | .50-3.0 | — |
| Magnesium, % | .21-.60 | .20-.50 | .26-1.0 | .15-.50 | .30-.80 | .30-1.0 | — |
| Sulfur, % | — | — | — | .15-.40 | .20-.30 | .26-.50 | .15-.20 |
| Boron, ppm | 4-25 | 1-10 | 21-55 | 5-10 | 20-50 | 30-80 | — |
| Copper, ppm | 6-20 | 2-7 | 10-30 | 5-25 | 10-50 | 7-30 | — |
| Iron, ppm | 21-250 | 65-100 | 51-350 | 50-150 | 100-350 | — | — |
| Manganese, ppm | 20-150 | 8-190 | 21-100 | 25-100 | 100-350 | 31-100 | — |
| Zinc, ppm | 20-70 | 15-30 | 21-50 | 15-70 | 20-50 | < 20-50 | — |
| *Adapted from Soil Fertility Handbook, Oklahoma State University. | |||||||
| Table 2. General symptoms of nutrient deficiency in plants. | |
| Nitrogen: Plant light green, lower leaves yellow to light brown, stalks short and slender, plants stunted. | Iron: Young leaves are chlorotic, with principal veins typically green; stalks short and slender. |
| Phosphorus: Plants dark green, often developing red and purple pigments; lower leaves sometimes yellow; plants stunted. | Zinc: Leaf spots on older leaves, with spots rapidly enlarging and generally involving the area between the veins; thick leaves; stalks with shortened internodes. |
| Potassium: Spots of dead tissue, usually at the tips and between the veins; marked margins of leaves. | Boron: Young leaves of the terminal bud are light green at the base; the bud eventually dies. |
| Magnesium: Mottled or chlorotic leaves, which typically redden; leaf tips and margins turned or cupped upward. | Copper: Young leaves are permanently wilted, with spotty or marked chlorosis. |
| Calcium: Young leaves of terminal bud hooded; with severe deficiency, dying buds; dying back at the tips and margins of the leaf. | Manganese: Spots of dead tissue scattered over the leaf; smallest veins tend to remain green. |
| Sulfur: In young leaves, veins and tissue between veins are light green. | |
| Table 3. Tissue sampling techniques for specific plants. | |||
| FIELD CROPS | |||
| Crop | When to sample | Where to sample | Number to sample |
| Alfalfa | Early bloom | Top 6 inches or upper third of plant | 12-30 |
| Canola | Before seed set | Recently mature leaf | 60-70 |
| Clover | Before bloom | Upper 1/3 of plant | 30-40 |
| Corn/sweet corn | Seedling stage OR Before tasseling OR Tasseling to silking | All above-ground portions First fully developed leaf from the top of the plant Leaf opposite and below ear |
15-20 15-20 12-20 |
| Cotton | Full bloom | Recently mature leaf from main stem | 40-50 |
| Grasses/forage mixes | Stage of best quality (before seed emerges) | Upper 4 leaves | 30-40 |
| Peanuts | Before or at bloom | Recently mature leaves | 40-50 |
| Small grains (barley, oats, wheat, rye, rice) | Seedling stage Before heading | All above-ground portions 4 uppermost leaf blades | 25-40 25-40 |
| Sorghum (milo) | Before or at heading | 2nd leaf from top of plant | 20-30 |
| Soybeans | Before or at bloom | Recently mature, trifoliate leaves from the top of the plant | 20-30 |
| Sugar beets | Midseason | Recently mature leaf at center of whorl | 25-30 |
| Sunflowers | Before heading Recently mature leaf | Before heading Recently mature leaf | 20-30 |
| VEGETABLE CROPS | |||
| Crop | When to sample | Where to sample | Number to sample |
| Asparagus | Maturity | Fern, 18-30 inches above ground line | 10-30 |
| Beans | Seedling stage OR Before or at bloom | All above-ground portions Recently mature leaf | 20-30 20-30 |
| Broccoli | Before heading | Recently mature leaf | 12-20 |
| Brussels sprouts | Midseason | Recently mature leaf | 12-20 |
| Celery | Midseason | Outer petiole of recently mature leaf | 12-20 |
| Cucumbers | Recently mature leaf | 12-20 | |
| Head crops(cabbage, cauliflower) | Before heading | Recently mature leaf at center of whorl | 12-20 |
| Leaf crops(such as lettuce, spinach) | Midseason | Recently mature leaf | 12-20 |
| Melons | Before fruit set | Recently mature leaf | 12-20 |
| Peas | Before or at bloom | Leaves from 3rd node from top | 40-60 |
| Peppers | Midseason | Recently mature leaf | 25-50 |
| Potatoes | Before or at bloom | 3rd to 6th leaf from growing tip | 25-30 |
| Sweet potatoes | Midseason or before root enlargement | 3rd to 6th leaf from tip center OR Mature leaves | 20-30 25-35 |
| Root/bulb crops(such as carrots, beets, onions) | Midseason before root or bulb enlargement | Recently mature leaf | 20-30 |
| Tomatoes (field) | Midbloom | 3rd to 4th leaf from growing tip | 15-20 |
| Tomatoes (trellis or indeterminate) | Midbloom from 1st to 6th cluster stage | Petiole of leaf below or opposite top cluster | 2-20 |
| ORNAMENTALS AND FLOWERS | |||
| Crop | When to sample | Where to sample | Number to sample |
| Carnations | Newly planted Established | 4th to 5th leaf pair from base 5th to 6th leaf pair from base | 20-30 20-30 |
| Chrysanthemums | Before or at bloom | Top leaves on flowering stem | 20-30 |
| Ornamental trees and shrubs | Current year's growth | Recently mature leaf | 30-70 |
| Poinsettias | Before or at bloom | Recently mature leaf | 15-20 |
| Roses | At bloom | Recently mature compound leaf on flowering stem | 25-30 |
| Turf | Active growth | Leaf blades. Avoid soil contamination. | 2 cups |
| FRUIT AND NUT CROPS | |||
| Crop | When to sample | Where to sample | Number to sample |
| Apples, pears, almonds, apricots, cherries, prunes, plums | Midseason(June-July) | Leaves from current season's nonfruiting, nonexpanding spurs | 50-100 |
| Peaches and nectarines | Midseason (June-July) | Midshoot leaflets/leaves | 25-100 |
| Grapes | At bloom | Petioles or leaves adjacent to basal clusters at bloom | 50-100 |
| Pecans | Midseason | Midshoot leaflets/leaves | 25-60 |
| Pistachios | Mid- to late season (August) | Terminal leaflets from nonfruiting shoots | 25-60 |
| Raspberries | Midseason | Recently mature leaves from laterals of primocanes | 30-50 |
| Strawberries | Midseason | Recently mature leaves | 25-40 |
| Walnuts | (June-July) | Terminal leaflets/leaves from nonfruiting shoots | 25-40 |
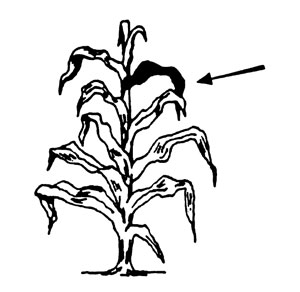
Corn before tasseling. Collect the first fully developed leaves from the top of 15-20 plants. If the plant is less than 12 inches tall, collect all of the above-ground portion.
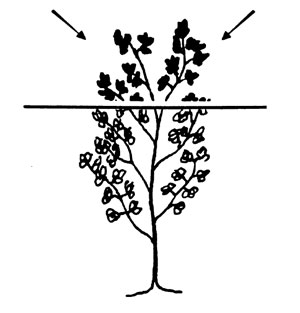
Alfalfa. Collect the top 6 inches or upper third of the plant at early bloom.
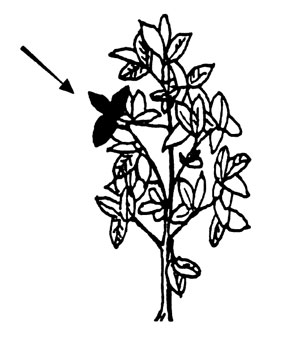
Soybeans. Collect recently mature trifoliate leaves from the top of 20-30 plants before or during bloom. (In the seedling stage, collect all of the above-ground portion of the plant.)
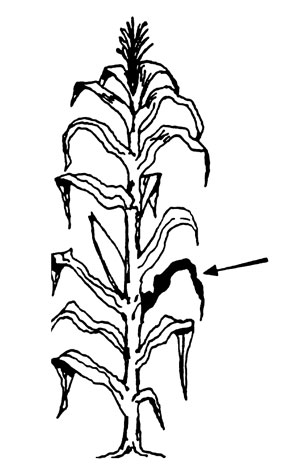
Corn from tasseling to silking. Collect the leaves below and opposite from the ear of 15-20 plants.
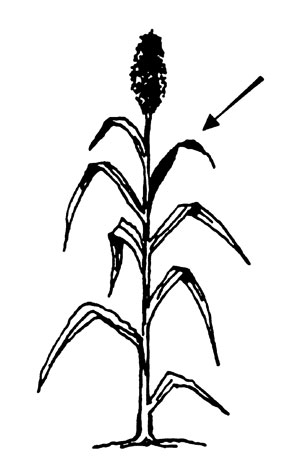
Sorghum. Collect the second leaf from the top of 20-30 plants before or at heading.
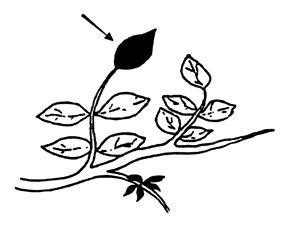
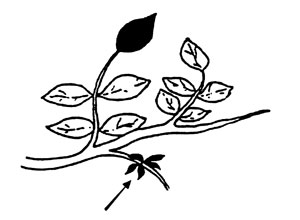
Pistachios and Walnuts. Collect terminal leaflets/s from nonfruiting shoots at mid- to late season.
Apples, Pears, Almonds, Apricots, Cherries, Prunes, Plums. Collect the leaves from the current season's nonfruiting, nonexpanding spurs at midseason.
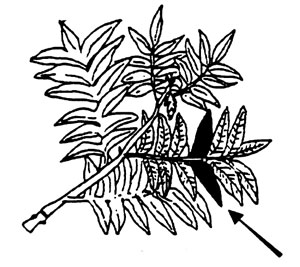
Pecans, Peaches, and Nectarines. Collect the midshoot leaflets/leaves at midseason.
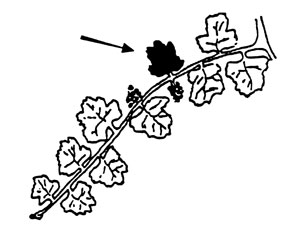
Grapes. Collect the petioles or leaves adjacent to basal clusters at bloom.
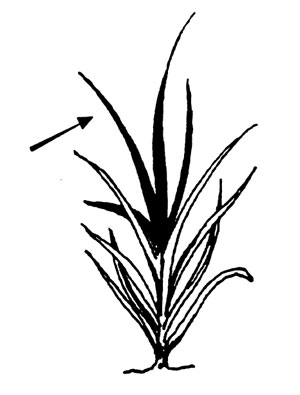
Small grains. Collect the four leaf blades from the top of 25-40 plants. Sample should equal 2 cups. (In the seedling stage, collect all of the above-ground portion.)
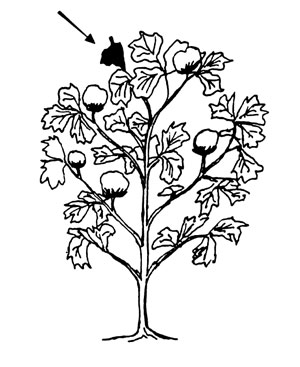
Cotton. Collect recent from the main stem on 40 to 50 plants selected at random at full bloom.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
July 1999, Las Cruces, NM


