Correlation of Climatic Factors and Occurrence of Puccinia grindeliae on Herbarium Specimens of Gutierrezia spp. Collected in Southwestern States Since 1891
Bulletin 773
C. M. Liddell, Assistant Professor of Plant Pathology, Department of Entomology, Plant Pathology and Weed Science
C. A. Waddell, Research Assistant, Department of Entomology, Plant Pathology and Weed Science
E. K. Haskins, Laboratory Assistant, Department of Entomology, Plant Pathology and Weed Science
J. P. McEntee, Former Research Assistant, present address Department of Microbiology, University of New Mexico School of Medicine
College of Agriculture, Consumer and Environmental Sciences New Mexico State University. (Print Friendly PDF)
Acknowledgements
This research was supported by the New Mexico Agricultural Experiment Station. We thank the curators of herbaria visited during the course of this study for their assistance in providing facilities and specimens. We particularly thank Dr. Kelly Allred (Department of Animal and Range Sciences) and Dr. Rich Spellenberg (Department of Biology), both of New Mexico State University, for their generous help with the study, particularly the identification of Gutierrezia species. We also thank Dr. Leigh Murray, Department of Experimental Statistics at New Mexico State University, for assistance with the statistical analyses and acknowledge Ted Slater’s excellent computer support.
Table of Contents
Procedures
Herbarium Collections
Survey of Herbarium Specimens
Analysis of Historical Weather Data
Field Survey
Results
Survey of Herbarium Specimens
Analysis of Historical Weather Data
Field Survey
Discussion
References
Broom snakeweed (Gutierrezia sarothrae [Pursh] Britton and Rusby) is a weedy, half-shrub native to the rangelands of the western United States, Mexico, and Canada (Lane, 1985). Gutierrezia sarothrae and related species have been spreading throughout southwestern rangelands over the past 100 years in response to environmental and human factors (McDaniel, Pieper, and Donart, 1982). Broom snakeweed is toxic to cattle and responsible for poor forage production (Heitschmidt, 1979; McDaniel et al., 1982; Nabado, Pieper, and Beck, 1980). We are evaluating the potential of Puccinia grindeliae Peck to act as a biological control agent of Gutierrezia spp. in the southwestern U.S.
We chose Puccinia grindeliae for this study because it is a member of a genus of plant pathogenic fungi that contains some of the most devastating known pathogens of plants and it attacks only broom snakeweed and closely related plants. Puccinia Persoon. species, many of which cause significant economic losses annually on crops throughout the world, are rust pathogens of virtually all higher plants (Cummins and Hiratsuka, 1983). Puccinia species are basidiomycetes, are therefore related to mushrooms and, in common with mushrooms, produce basidia and basidiospores. There are 3,000 to 4,000 different species of Puccinia, all showing a remarkable degree of host specificity. Most species of Puccinia attack only one or two plant genera and in some cases may attack only a few varieties of one plant species. This means they are potentially ideal biological control agents, being both devastating pathogens that are highly selective and highly damaging to target weeds but not to nontarget plants. Puccinia species often do not kill their hosts, but reduce photosynthesis dramatically by destroying leaf tissue and eliminating flowering and fruit production very effectively (fig. 1a).

Fig. 1a. Herbarium specimen #567 from Nara Visa, NM, collected in 1907.

Fig. 1b. Close-up of Puccinia grindeliae on specimen #567.
Rust fungi such as Puccinia are obligate pathogens of living land plants and get their name from the rusty yellow or orange color of the spore pustules on green plant tissue. Many species of Puccinia produce up to five distinct spore stages, and many require two unrelated groups of host plants in order to complete their life cycle (heteroecious life cycle). However, several species such as P. grindeliae produce only two spore stages and complete their life cycle on only one host (autoecious life cycle). Puccinia grindeliae produces only basidiospores and teliospores and completes its entire life cycle on Gutierrezia species and about 20 other closely related genera of weedy plants (Cummins, 1978). The basidiospores are the infectious spore stage, and the teliospores are dark, thick-walled overseasoning spores that occur in small black pustules called “telia” on leaves and photosynthetic stems. Occasionally, we have observed other spore stages, such as aeciospores and the rust-colored urediniospores, but these spore stages do not appear to be common in the field, and we do not believe they play an important role in the epidemiology of snakeweed rust caused by P. grindeliae.
In epidemiological studies on P. grindeliae (Liddell, Waddell, and McEntee, 1993), we found the examination of dried herbarium specimens of Gutierrezia spp. provided a good source of information on the historical distribution of P. grindeliae. This bulletin reports on the use of dried herbarium specimens to determine the historical distribution patterns of P. grindeliae, as part of a major project to determine the long-term potential of P. grindeliae as a biocontrol agent of broom snakeweed in the southwestern U.S.
Herbarium collections are a valuable resource for historical investigations into pathogens of non-crop plants such as Gutierrezia. First, it is easy to examine a large number of sites quickly and efficiently, covering a wider area than can be readily visited. Second, some collections represent sites that are no longer accessible or existent. Third and most important, dried herbarium specimens provide the only source of historical information on diseases of rangeland weeds, due to an almost complete lack of published information on these pathogens.
P. grindeliae was first described in Colorado on Grindeliae squarrosa Pursh (Dunal) in 1879 (Peck, 1879). Most of the information about diseases and pathogens of rangeland weeds, such as Gutierrezia, is contained only in descriptive reports and floras. P. grindeliae has been reported in just 14 publications since 1918 (Brenckle, 1918; Cummins, 1979; Farr, Bills, Chamuris, and Rossman, 1989; Gilbertson and McHenry, 1969; Solheim, 1934, 1940, 1943, 1954; Solheim and Cummins, 1957, 1959, 1970a, 1970b, 1979; Yohem, Cummins, and Gilbertson, 1985). The scant epidemiological and ecological data available on P. grindeliae is found only in detailed floras, such as Cummins (1978). There are certainly no reports on the distribution of this pathogen in the first half of this century, and virtually nothing is known about its occurrence. This lack of information on diseases and pathogens of rangeland weeds is due to their low economic importance, coupled with the nature of the habitats where these plants occur.
The objectives of our study are: 1) to provide some rudimentary information on the occurrence of P. grindeliae over the past 100 years; 2) to determine the longevity of P. grindeliae at specific sites in New Mexico and Arizona; 3) to correlate rust collections with climate records; and 4) to evaluate the correlation of herbarium collections with occurrence in the field and mortality of snakeweed over the past 100 years.
Procedures
Herbarium Collections
Dried herbarium specimens of 11 species of Gutierrezia at nine university herbaria in Arizona, New Mexico, and Texas (table 1) were examined microscopically for telia of P. grindeliae. The study comprised a total of 1,048 herbarium specimens of Gutierrezia spp. collected throughout the southwestern U.S. from 1891 to 1991. Most of these specimens were collected in North America, and all identities were confirmed by Meredith Lane (Lane, 1982, 1983, 1985) (table 1). Seven additional specimens of P. grindeliae, on G. sarothrae and G. californica, were also examined at the University of Arizona Mycology Herbarium (table 1). All herbarium specimens examined were originally assigned to 24 species of Gutierrezia, which were reduced to synonymy with 11 species based on a recent taxonomic revision (Lane, 1985). Accepted species included in our study are listed in table 2.
Table 1. Herbarium collections examined for the occurrence of Puccinia grindeliae telia on Gutierrezia spp.
| Herbarium | Number of specimens examined |
Number of specimens bearing telia of P. grindeliae |
| New Mexico State University Biological Science Range Science |
162 25 |
14 2 |
| University of New Mexico Biology |
238 |
4 |
| Texas A & M University Range Science |
241 |
4 |
| University of Texas at Austin Botany |
252 |
9 |
| University of Texas at El Paso Biology |
36 |
1 |
| Texas Tech University Range Science |
36 |
0 |
| University of Arizona Biological Sciences |
58 |
5 |
| Sub-Total | 1048 | 39 |
| University of Arizona Mycologya |
7 |
7 |
| Total | 1055 | 46 |
| aHerbarium collections of P. grindeliae | ||
Table 2. Species of Gutierrezia herbarium and field survey specimens examined for the presence of Puccinia grindeliae.
| Species |
Number of |
Number of herbarium specimens bearing telia of P. grindeliae |
Number of field survey specimens examined |
Number of field survey specimens bearing telia of P. grindeliae |
| Gutierrezia Lagasca spp. | 11 | 0 | 0 | 0 |
| G. alamanii A. Gray | 2 | 0 | 0 | 0 |
| G. californica (D.C) Torrey & A. Gray | 10 | 3a | 0 | 0 |
| G. conoidea (Hemsley) M.A. Lane | 2 | 0 | 0 | 0 |
| G. grandis S.F. Blake | 2 | 0 | 0 | 0 |
| G. mandonii (Sch. Bip.) Solbrig | 10 | 0 | 0 | 0 |
| G. microcephala (D.C) A. Gray | 201 | 4 | 0 | 0 |
| G. sarothrae (Prush) Britton & Rusby | 652 | 35b | 35 | 20 |
| G. serotina E. Greene | 7 | 1 | 0 | 0 |
| G. sphaerocephala A. Gray | 82 | 2 | 0 | 0 |
| G. texana (D.C) Torrey & A. Gray | 58 | 1 | 0 | 0 |
| G. wrightii A. Gray | 18 | 0 | 0 | 0 |
| aThese three specimens of Puccinia grindeliae were from the University of Arizona mycological herbarium. bIncludes four specimens of Puccinia grindeliae from the University of Arizona mycological herbarium. |
||||
Survey of Herbarium Specimens
Herbarium specimens were examined in a systematic fashion by looking quickly for evidence of Puccinia grindeliae telia on leaves and stems. If no obvious telia were found the specimen was examined closely using a 10× lens for 5 minutes. All telia were confirmed to be P. grindeliae by removing a small number of teliospores from each specimen for microscopic observation. Annotations were made on all specimens where the presence of P. grindeliae was confirmed. The location of each collection and date of collection was noted for each specimen, and each location was mapped as accurately as annotations on the herbarium sheet would allow.
Analysis of Historical Weather Data
All specimens from herbaria, except the seven collections of P. grindeliae from the University of Arizona Mycology Herbarium, were mapped to obtain elevation, latitude, and longitude and to locate the nearest weather stations for precipitation and temperature data (table 3). Weather data were obtained from the National Oceanic and Atmospheric Administration, National Climatic Center (1905–1991); Williams and McAllister (1981); and Williams (1986). Elevation data were obtained from the herbarium sheets or from high-resolution topographic maps. Due to poor annotation on herbarium sheets and the paucity of good historic weather data, only 46 sites (23 paired sites) could be used in this analysis (table 3).
Table 3. Specimens used for logistic regression analysis.
| Specimen # |
Rust +/ − |
Species | County | Date | Weather station |
Distance from weather station (km) |
Elevation of site (m) |
Elevation of weather station (m) |
| 81 | + | G. sarothrae | Otero | 09 Aug 39 | Mescalero | 18.6 | 2092 | 2020 |
| 670 | − | G. sarothrae | Otero | 14 Sep 60 | Mescalero | 9.3 | 2234 | 2020 |
| 121 | + | G. sarothrae | Otero | 08 Sep 39 | Mescalero | 18.6 | 2092 | 2020 |
| 795 | − | G. sarothrae | Otero | 05 Sep 71 | Mescalero | 3.5 | 2084 | 2020 |
| 518 | + | G. microcephala | Lincoln | 07 Oct 07 | Ft. Stanton | 34.8 | 1966 | 1900 |
| 698 | − | G. sarothrae | Lincoln | 09 Oct 71 | Ft. Stanton | 32.5 | 1755 | 1900 |
| 519 | + | G. microcephala | Quay | 18 Sep 07 | Tucumcari | 55.7 | 1167 | 1280 |
| 300 | − | G. sarothrae | Quay | 26 Sep 64 | Tucumcari | 18.6 | 1210 | 1280 |
| 529 | + | G. sarothrae | Doña Ana | 28 Oct 06 | Mesilla Pk | 23.2 | 1314 | 1067 |
| 591 | − | G. sphaerocephala | Doña Ana | 16 Sep 03 | Mesilla Pk | 23.2 | 1309 | 1067 |
| 547 | + | G. sarothrae | Quay | 19 Oct 07 | Tucumcari | 23.2 | 1321 | 1280 |
| 531 | − | G. sarothrae | Quay | 24 Sep 07 | Tucumcari | 1.2 | 1247 | 1280 |
| 550 | + | G. sarothrae | San Miguel | 28 Sep 07 | Las Vegas | 1.2 | 1981 | 1951 |
| 845 | − | G. microcephala | San Miguel | 24 Jul 63 | Las Vegas | 41.8 | 2300 | 1951 |
| 557 | + | G. sarothrae | Colfax | 22 Sep 07 | Springer | 1.6 | 1768 | 1786 |
| 307 | − | G. sarothrae | Colfax | 05 Aug 44 | Springer | 1.6 | 1771 | 1786 |
| 561 | + | G. sarothrae | De Baca | 11 Oct 07 | Portales | 78.9 | 1253 | 1220 |
| 571 | − | G. sarothrae | De Baca | 28 Aug 73 | Ft. Sumner | 11.6 | 1311 | 1207 |
| 565 | + | G. sarothrae | Bernalillo | * 1936 | Albuquerque | 23.2 | 1806 | 1585 |
| 657 | − | G. sarothrae | Bernalillo | 11 Sep 64 | Albuquerque | 27.8 | 2012 | 1585 |
| 587 | + | G. sphaerocephala | Sierra | 20 Sep 07 | Hillsboro | 23.2 | 1615 | 1593 |
| 303 | − | G. sarothrae | Luna | 06 Sep 39 | Deming | 27.8 | 1448 | 1319 |
| 689 | + | G. sarothrae | Otero | 02 Sep 52 | Ruidoso | 21.0 | 2042 | 2096 |
| 671 | − | G. sarothrae | Otero | 10 Oct 71 | Ruidoso | 19.5 | 2164 | 2096 |
| 918 | + | G. sarothrae | Guadelupe | 15 Sep 35 | Santa Rosa | 2.4 | 1448 | 1410 |
| 294 | − | G. sarothrae | Guadelupe | 03 Sep 29 | Santa Rosa | 1.6 | 1394 | 1410 |
| 937 | + | G. sarothrae | Otero | 15 Aug 38 | Alamogordo | 9.3 | 2235 | 1311 |
| 243 | − | G. sarothrae | Otero | 09 Oct 16 | Alamogordo | 18.6 | 1341 | 1311 |
| 723 | + | G. sarothrae | Sandoval | 25 Sep 31 | Santa Fe | 32.5 | 1608 | 2134 |
| 820 | − | G. sarothrae | Sandoval | 08 Jun 75 | Santa Fe | 27.8 | 1676 | 2134 |
| 759 | + | G. sarothrae | Mora | 01 Sep 76 | Springer | 51.0 | 2396 | 1786 |
| 926 | − | G. sarothrae | Colfax | 08 Sep 32 | Springer | 11.7 | 1829 | 1786 |
| 567 | + | G. sarothrae | Quay | 02 Oct 07 | Nara Visa | 1.6 | 1265 | 1279 |
| 554 | − | G. sarothrae | Union | 09 Oct 07 | Albert | 37.1 | 1681 | 1433 |
| 546 | + | G. sarothrae | Quay | 21 Oct 07 | Tucumcari | 1.6 | 1244 | 1280 |
| 531 | − | G. sarothrae | Quay | 24 Sep 07 | Tucumcari | 1.6 | 1247 | 1280 |
| 555 | + | G. sarothrae | Santa Fe | 28 Sep 07 | Santa Fe | 27.8 | 1859 | 2134 |
| 646 | − | G. sarothrae | Santa Fe | 01 Oct 32 | Santa Fe | 2.4 | 2179 | 2134 |
| 570 | + | G. sarothrae | Lincoln | 03 Oct 07 | Ft. Stanton | 32.5 | 1966 | 1900 |
| 588 | − | G. sphaerocephala | Lincoln | 23 Jul 73 | Carrizozo | 27.8 | 2012 | 1658 |
| 683 | + | G. sarothrae | Mora | 01 Aug 76 | Ocate | 9.3 | 2396 | 2198 |
| 954 | − | G. sarothrae | Taos | 10 Aug 66 | Taos | 25.6 | 2258 | 2129 |
| 1071 | + | G. sarothrae | Lincoln | 03 Aug 80 | Capitan | 9.0 | 1905 | 1935 |
| 709 | − | G. sarothrae | Lincoln | 01 Oct 71 | Ft. Stanton | 15.0 | 2128 | 1900 |
| 1078 | + | G. microcephala | Lincoln | 02 Oct 83 | Capitan | 51.0 | 1496 | 1935 |
| 805 | − | G. sarothrae | Lincoln | 01 Aug 49 | Capitan | 11.7 | 2012 | 1935 |
| * Specimen #565 was collected in the summer of 1936 according to the original herbarium data sheet. | ||||||||
The relationship between observing rust on herbarium specimens and weather data at the date of specimen collection was analyzed using logistic regression (SAS Institute, 1989). The dependent variable was the score for presence of rust (+ or −), based on paired specimens collected at the same or nearby sites in different years. The independent variables were quarterly rainfall totals and mean daily temperatures for the 12 quarters preceding the date of collection.
Because P. grindeliae is an obligate pathogen, conditions that favor the host may be expected to favor the pathogen. Heitschmidt (1979) showed the relative abundance of Gutierrezia spp. in Texas was correlated with average daily maximum temperature in April and precipitation in May for the year of collection. Therefore, logistic regression models using monthly rainfall and monthly maximum temperatures for each of the 12 months preceding the date of collection were fitted to determine whether rust observation on herbarium specimens correlated with Heitschmidt’s model for Gutierrezia occurrence.
The basic assumption in these analyses was that the probability of collecting a rusted plant was directly proportional to the number of rusted plants in the collection area. Only data from herbarium collections of Gutierrezia spp. (table 1) were used in this analysis to ensure random sampling of rust occurrence. All rusted and non-rusted herbarium specimens used in the logistic regression analysis except one (specimen 820: table 4) were collected in autumn between the months of August and October. Specimen 820 was collected in the spring (April 8, 1975).
Collections made during the 1990–1993 field survey and held in the NMSU Plant Pathology Herbarium (table 4), and specimens of P. grindeliae in the Mycology Herbarium at the University of Arizona (table 1) were excluded from the logistic regression analysis.
Table 4. Specimens cited and their origins.
| ID # | Herbarium | Herbarium # | Species | Specific location |
| 81 | TAMU | 39232 | G. sarothrae | 3 miles SW of Ruidoso Jct., Lincoln Co. NM |
| 121 | TAMU | 39169 | G. sarothrae | 3 miles SW of Ruidoso Jct., Lincoln Co. NM |
| 243 | UTA | NA | G. sarothrae | South of Dog Canyon, Otero Co. NM |
| 294 | UTA | 147577 | G. sarothrae | Santa Rosa, Guadalupe Co. NM |
| 300 | UTA | 233494 | G. sarothrae | 15 miles E of Tucumcari, Quay Co. NM |
| 302 | UTA | 147558 | G. sarothrae | 20 miles SW of Clayton, Union Co. NM |
| 303 | UTA | NA | G. sarothrae | 21 miles NW of Deming, Luna Co. NM |
| 307 | UTA | 147715 | G. sarothrae | 1/2 mile E of Springer, Colfax Co. NM |
| 518 | NMSUBIO | 30382 | G. sarothrae | White Oaks, Lincoln Co. NM |
| 519 | NMSUBIO | 30384 | G. microcephala | Endee, Quay Co. NM |
| 529 | NMSUBIO | 39007 | G. sarothrae | Plains near Doña Ana Mountains, Doña Ana Co. NM |
| 531 | NMSUBIO | 30443 | G. sarothrae | Tucumcari, Quay Co. NM |
| 546 | NMSUBIO | 30385a | G. sarothrae | Ogle, Quay Co. NM |
| 547 | NMSUBIO | 30385b | G. sarothrae | Tucumcari, Quay Co. NM |
| 550 | NMSUBIO | 39058 | G. sarothrae | E. of Las Vegas, San Miguel Co. NM |
| 554 | NMSUBIO | 30426 | G. sarothrae | Beenham, Union Co. NM |
| 557 | NMSUBIO | 30425 | G. sarothrae | Springer, Colfax Co. NM |
| 561 | NMSUBIO | 30418 | G. sarothrae | La Lande, De Baca Co. NM |
| 565 | NMSUBIO | USDA | G. sarothrae | Mesa E. of Albuquerque, Bernalillo Co. NM |
| 567 | NMSUBIO | 30362 | G. sarothrae | Nara Visa, Quay Co. NM |
| 570 | NMSUBIO | 30383 | G. sarothrae | White Oaks, Lincoln Co. NM |
| 571 | NMSUBIO | 7436 | G. sarothrae | 9 miles NNE of Ft. Sumner T.4 N, R.26.E, sec. 3, De Baca Co. NM |
| 587 | NMSUBIO | 30402 | G. sphaerocephala | Lake Valley, Sierra Co. NM |
| 588 | NMSUBIO | 43782 | G. sphaerocephala | 6 miles NW of Jicarilla and 1/4 mile S. of Ancho, Lincoln Co. NM |
| 591 | NMSUBIO | 30403 | G. sphaerocephala | Lake E. of Doña Ana Mts., Doña Ana Co. NM |
| 594 | NMSUBIO | 30409 | G. sphaerocephala | Mesa near Las Cruces, Doña Ana Co. NM |
| 646 | UNM | 1842 | G. sarothrae | Road side near Santa Fe, Santa Fe Co. NM |
| 657 | UNM | 43424 | G. sarothrae | Embudo Canyon, Sandia Mt., Cibola Nat. For. NE 1/4 S.3, T.10 N, R.4 E, Bernalillo Co. NM |
| 670 | UNM | 23972 | G. sarothrae | 6 miles NE of Mescalero Rt. 70 in a valley, Otero Co. NM |
| 671 | UNM | 51451 | G. sarothrae | Hwy. 70 1 mile east of Mescalero, Otero Co. NM |
| 683 | UNM | 68982 | G. sarothrae | Les Felris canyon, N. car camp area of Lazy 3 Ranch, 7 miles W. of Ocata, Mora Co. NM |
| 689 | UNM | 68982 | G. sarothrae | Roadside Mescalero, Otero Co. NM |
| 698 | UNM | 51185 | G. sarothrae | Roadside Hwy. 380 4 miles E. of Carrizozo, Lincoln Co. NM |
| 709 | UNM | 51195 | G. sarothrae | Roadside Hwy. 37 1 mile N. of Angus, Lincoln Co. NM |
| 723 | UNM | 941 | G. sarothrae | Roadside near Domingo, Sandoval Co. NM |
| 759 | UNM | 59607 | G. sarothrae | Les Felris canyon, N. car camp area of Lazy 3 Ranch, 7 miles W. of Ocata, Mora Co. NM |
| 769 | UNM | 29223 | G. sarothrae | Near Stallion site on US 380, Socorro Co. NM |
| 795 | UNM | 51493 | G. sarothrae | Roadside Hwy. 70 15 miles W. of Ruidoso, Otero Co. NM |
| 805 | UNM | 10405 | G. sarothrae | 0.6 miles S. of Nogal, Lincoln Co. NM |
| 820 | UNM | 57196 | G. sarothrae | Cochiti Lake at the Jct. of Blend Canyon and Rio Grande, Sandoval Co. NM |
| 845 | UNM | 67814 | G. microcephala | S. of Glorieta Mesa SS T.13N, R.13E, San Miguel Co. NM |
| 883 | NMSUEPWS | EP0001 | G. sarothrae | Alter rd. off Sasabe rd., AZ Hwy. 286 W. of Tuson, Pima Co. AZ |
| 884 | NMSUEPWS | EP0002 | G. sarothrae | Buenas Aires Ranch N. of Sasabe, AZ, Pima Co. AZ |
| 885 | NMSUEPWS | EP0003 | G. sarothrae | Buenas Aires Ranch N. of Sasabe, AZ, Pima Co. AZ |
| 886 | NMSUEPWS | EP0004 | G. sarothrae | Buenas Aires Ranch N. of Sasabe, AZ, Pima Co. AZ |
| 887 | NMSUEPWS | EP0005 | G. sarothrae | Youngblood Ranch near cattle pond 1, west road, Socorro Co. NM |
| 888 | NMSUEPWS | EP0006 | G. sarothrae | Youngblood Ranch near fence, Socorro Co. NM |
| 889 | NMSUEPWS | EP0007 | G. sarothrae | Las Cruces Airport, Doña Ana Co. NM |
| 890 | NMSUEPWS | EP0008 | G. sarothrae | Jornada Exp. Range South windmill, Doña Ana Co. NM |
| 891 | NMSUEPWS | EP0009 | G. sarothrae | Jornada Exp. Range South windmill, Doña Ana Co. NM |
| 892 | NMSUEPWS | EP0081 | G. sarothrae | Jct. 22 Santo Domingo Hwy., Sandoval Co. NM |
| 893 | NMSUEPWS | EP0082 | G. sarothrae | Nara Visa, Quay Co. NM |
| 897 | NMSUEPWS | EP0016 | G. sarothrae | 1/10 of a mile S. of Tucson, AZ, Pima Co. AZ. |
| 898 | NMSUEPWS | EP0017 | G. sarothrae | N. of Lovington E of the power plant, Hidalgo Co. NM |
| 899 | NMSUEPWS | EP0018 | G. sarothrae | Rd to Hachita, AZ mile marker 15, Pima Co. AZ |
| 900 | NMSUEPWS | EP0019 | G. sarothrae | Youngblood Ranch, Socorro Co. NM |
| 901 | NMSUEPWS | EP0020 | G. sarothrae | Youngblood Ranch, Socorro Co. NM |
| 902 | NMSUEPWS | EP0021 | G. sarothrae | Rt. 380 7/10 mile E. of mile marker 180, Chavez Co. NM |
| 903 | NMSUEPWS | EP0022 | G. sarothrae | Rt. 380 7/10 mile E. of mile marker 180, Chavez Co. NM |
| 904 | NMSUEPWS | EP0023 | G. sarothrae | 15–16 mile NE of San Antonio @ gate of Youngblood Ranch, Socorro Co. NM |
| 905 | NMSUEPWS | EP0024 | G. sarothrae | Youngblood Ranch, Socorro Co. NM |
| 906 | NMSUEPWS | EP0025 | G. sarothrae | S. of Deming 5.2 miles E. of Rockhound St. Park access rd. S. side of rd., Luna Co. NM |
| 907 | NMSUEPWS | EP0026 | G. sarothrae | S. of Deming 5.2 miles E. of Rockhound St. Park access rd. S. side of rd., Luna Co. NM |
| 908 | NMSUEPWS | EP0027 | G. sarothrae | S. of Deming 5.2 miles E. of Rockhound St. Park ccess rd. S. side of rd., Luna Co. NM |
| 909 | NMSUEPWS | EP0028 | G. sarothrae | S. of Deming 5.2 miles E. of Rockhound St. Park access rd. S. side of rd., Luna Co. NM |
| 910 | NMSUEPWS | EP0029 | G. sarothrae | NE of Deming on US 180 (15.2 miles from I-10) W. side of rd. |
| 918 | UABIO | G. sarothrae | Santa Rosa, Tucumcari Hwy., Guadalupe Co. NM | |
| 926 | UABIO | 138281 | G. sarothrae | Roadside 8 miles S. of Springer, Colfax Co. NM |
| 937 | UABIO | 105609 | G. sarothrae | Head of Dry Canyon, Otero Co. NM |
| 949 | UABIO | 181084 | G. sarothrae | Jornada Exp. Range, 17 miles N. of Las Cruces, Doña Ana Co. NM |
| 954 | UABIO | 174552 | G. sarothrae | Near Picurio Pueblo in valley of Rio Pueblo, Taos Co. NM |
| 963 | UABIO | 102635 | G. sphaerocephala | Ranch 23 miles N. of Las Cruces, Doña Ana Co. NM |
| 1069 | NMSURS | W#7 | G. microcephala | 1/4 mile E. of A Mountain in Las Cruces, Doña Ana Co. NM |
| 1071 | NMSURS | LE299 | G. sarothrae | Fort Stanton, short duration pasture, Lincoln Co. NM |
| 1078 | NMSURS | LO402 | G. microcephala | R 19 E, T 75, sec 29, NW1/4 of NW1/4 4.6 miles W. Middle Arroyo Ranch Road on NM 48, Lincoln Co. NM |
| 1093 | NMSUEPWS | EP0083 | G. sarothrae | Forest rd. 90, Lincoln Nat. Forest, W side of road about 3 1/2 miles from NM Hwy. 82, Otero Co. NM |
| 1094 | NMSUEPWS | EP0084 | G. sarothrae | 2.8 miles from corner of 4th and Maxwell, E. of Springer N. side Rt. 56, Colfax Co. NM |
| 1095 | NMSUEPWS | EP0086 | G. sarothrae | Mile marker 259 N side of the Hwy. 2 miles from Ruidoso Jct., Lincoln Co. NM |
| 1096 | NMSUEPWS | EP0087 | G. sarothrae | Point of interest Mescalero, Otero Co. NM |
| Key to Herbaria NMSUBIO: New Mexico State University—Biology Herbarium NMSUEPWS: New Mexico State University—Plant Pathology Herbarium NMSURS: New Mexico State University—Range Science Herbarium TAMU: Texas A&M University—Range Science Herbarium UABIO: University of Arizona—Biological Sciences Herbarium UNM: University of New Mexico—Biology Herbarium UTA: University of Texas at Austin—Botany Herbarium |
||||
Field Survey
A series of field surveys of Gutierrezia species conducted from 1990 to 1993 had two primary objectives. First, sites represented by rusted herbarium specimens that could be located accurately from annotations on the herbarium sheet were visited to determine the current status of the host and pathogen. Second, random surveys were conducted to determine, as fully as possible, the extant distribution of Puccinia grindeliae in New Mexico.
Only 13 collection sites of rusted herbarium specimens of G. sarothrae from New Mexico could be located accurately from annotations on the herbarium sheets. These were revisited in 1990–1993 (table 5). Only one non-rusted herbarium collection was located accurately and revisited (table 5). Because it is impossible to determine population densities of hosts and pathogens from herbarium specimens, this aspect of the survey was designed to gather data only on the presence or absence of the host and pathogen presently at each site.
Table 5. Herbarium specimen collection sites revisited during the field survey.
| Herbarium specimen ID # |
Revisitation classa |
Collection date |
Rust presence |
Survey specimen ID # |
Collection date |
Rust presence |
| 81 | I | 8 Sep 07 | + | 1095 | 8 Oct 93 | + |
| 121 | I | 8 Sep 07 | + | 1095 | 8 Oct 93 | + |
| 302 | II | NA | + | 1 Jul 91 | − | |
| 550 | II | 22 Sep 07 | + | 4 Jul 91 | − | |
| 555 | II | 28 Sep 07 | + | 4 Jul 91 | − | |
| 557 | II | 22 Sep 07 | + | 1094 | 29 Jul 93 | + |
| 567 | II | 2 Oct 07 | + | 889 | 15 Jul 91 | + |
| 689 | II | 2 Sep 52 | + | 1096 | 8 Oct 93 | + |
| 723 | II | 25 Sep 31 | + | 892 | 3 Jul 91 | + |
| 769 | I | 19 Sep 61 | − | 887 | 20 Apr 90 | + |
| 937 | II | 15 Aug 38 | + | 1093 | 6 Jul 93 | + |
| 1071 | II | 3 Aug 80 | + | 10 Oct 93 | − | |
| 1078 | I | 2 Oct 83 | + | 10 Oct 93 | − | |
| a Revisitation class I—excellent annotation on herbariums sheet allowing location of original collection site to within 500 meters; Revisitation class II—reasonable annotation on herbarium sheets allowing location of original collection site to within 2–3 km. |
||||||
Random surveys were conducted throughout New Mexico by driving along roads in 20 of 33 counties and stopping to examine all major communities of G. sarothrae for the presence of P. grindeliae. At least 200 individual G. sarothrae communities were examined in the random survey. The survey procedure was the same for both surveys. At each site the observer walked straight toward the middle of the G. sarothrae community for 100 meters and then curved back to the road along a wide arc. Every 20 meters, the observer closely examined a small number of potentially rusted plants for 5 minutes. At each site, the observer examined no fewer than 10 plants for telia of P. grindeliae before moving on to a new site.
Preliminary identification of P. grindeliae was made in the field. Collections were transported to the laboratory in paper bags and plant presses for confirmation of both host and pathogen identities and permanent mounting.
Results
Survey of Herbarium Specimens
Overall, 3.7% of 1,048 Gutierrezia spp. herbarium specimens were rust positive (table 1). The oldest specimen was collected in 1891 and the earliest rusted specimen was from the Jornada Experimental Range near Las Cruces, NM, in 1906 (specimen 529). The condition of these old specimens was generally very good. Of 488 specimens examined from New Mexico, 24 were rust positive: 12 between 1891 and 1910, none between 1910 and 1930, six between 1931 and 1950, one between 1951 and 1970, four between 1971 and 1990; one rust-positive collection was not dated. Based on these observations, 4.9% of herbarium specimens collected in New Mexico between 1891 and 1990 were rusted (table 6). The 24 diseased herbarium specimens collected from New Mexico were found in 14 counties ranging in elevation from 1100 m to 2500 m located east of the Rio Grande (fig. 2).
![]() ) and non-rusted field survey specimens (
) and non-rusted field survey specimens (![]() ) are also shown. The large shaded circles represent sites that were revisited in 1990-1993 (table 5)." width="500" height="292" />
) are also shown. The large shaded circles represent sites that were revisited in 1990-1993 (table 5)." width="500" height="292" />
Fig. 2. Map of Arizona and New Mexico showing sites where herbarium specimens diseased with Puccinia grindeliae were collected from 1891 to 1990 (●) and sites where the field survey collection of diseased plants were made (◙). Collection sites of non-rusted herbarium specimens (○) and non-rusted field survey specimens (□) are also shown. The large shaded circles represent sites that were revisited in 1990–1993 (table 5).
Of the 119 herbarium specimens from Arizona, 5 were rust positive: one between 1911 and 1930, two between 1931 and 1950, and two between 1971 and 1990. Based on these specimens, 4.2% of plants collected in Arizona from 1911 to 1990 were diseased.
Of the seven specimens of P. grindeliae held at the University of Arizona Mycology Herbarium, five were collected in Arizona, and two were collected in Colorado and Wyoming. Overall, the ten diseased specimens from Arizona were collected from seven counties spanning the state from north to south at elevations ranging from 750 m to 2100 m (fig. 2). Diseased herbarium specimens were collected from other parts of the U.S.: California (1 diseased specimen out of 45), Colorado (0/27), Nevada (1/23), Nebraska (1/1), Texas (1/235), Utah (1/39), and Wyoming (1/8) (fig. 3). Four diseased specimens were also collected from Mexico (4/27) (table 6).
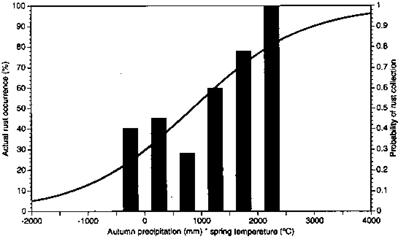
Fig. 3. Plot of the probability of any herbarium specimen being diseased with Puccinia grindeliae based on the product of the precipitation (mm) of the fall quarter in which the specimen was collected and the mean daily temperature (°C) of the preceding spring quarter (right axis). Actual percent rust occurrence on herbarium specimens based on 23 paired sites (table 3) is shown as bars (left axis).
Table 6. States in the U.S. and other countries where specimens were collected.
| State | Number of herbarium specimens examined |
Number of herbarium specimens bearing telia of P. grindeliae |
Number of field survey specimens examined |
Number of field survey specimens bearing telia of P. grindeliae |
| Argentina | 10 | 0 | 0 | 0 |
| Canada Saskatchewan |
3 2 |
0 0 |
0 0 |
0 0 |
| Mexico | 27 | 4 | 0 | 0 |
| United States Arizona California Colorado Idaho Kansas Nebraska Nevada New Mexico North Dakota Oklahoma Oregon South Dakota Texas Utah Wyoming |
1008 119 45 27 5 5 1 23 488 3 8 1 1 235 39 8 |
35 10a 1 1b 0 0 1 1 24 0 0 0 0 1 1 2c |
35 6 0 0 0 0 0 0 29 0 0 0 0 0 0 0 |
20 0 0 0 0 0 0 0 20 0 0 0 0 0 0 0 |
| a Includes five specimens of P.grindeliae from the University of Arizona Mycology Herbarium. b This specimen of P. grindeliae was from the University of Arizona Mycology Herbarium. c Includes one specimen of P. grindeliae from the University of Arizona Mycology Herbarium. |
||||
Analysis of Historical Weather Data
Logistic analysis of historical weather data showed the probability of collecting a diseased specimen was positively correlated
χ2 = 4.23[p ≤ 0.03]
with the product of precipitation in the quarter of collection and mean temperature in the preceding spring quarter. The logistic regression equation for the relationship between autumn precipitation (mm) (AUPRECIP), spring temperature (°C) (SPTEMP), and probability of rust occurrence used was:
ln(p/[1 − p]) = − 0.8706 + 0.00103 * (AUPRECIP * SPTEMP).
Solving for p in this equation, the predicted probability (p) of any herbarium specimen being diseased by P. grindeliae is:
p = (1 + exp [0.8706 − 0.00103 * (AUPRECIP * SPTEMP)]) − 1.
The graph of the predicted probability (p) as given by this equation is shown in fig. 3. This indicates the predicted probability of a herbarium specimen being diseased with P. grindeliae is higher in years when both autumn precipitation and spring temperature are high.
Field Survey
In most cases, Gutierrezia populations were still abundant at the herbarium collection sites. A few areas have been subject to highway development and roadside burning for many years, and Gutierrezia spp. were present in low numbers. Although the herbarium survey of Gutierrezia communities in Arizona disclosed 10 rusted specimens, no rusted specimens were found during 1990–93 field surveys. Field surveys of New Mexico communities of Gutierrezia yielded 20 rusted and nine non-rusted specimens of G. sarothrae collected at elevations from 1100 m to 1800 m. These specimens were permanently mounted for the New Mexico State University Plant Pathology Herbarium and given accession numbers as shown in table 4. In 1990–1993 Puccinia grindeliae was found in only eight of the 13 sites, which were located accurately from annotations on the herbarium sheets. There was only one site where no rust was present on the herbarium specimen,but P. grindeliae was present in 1990–1993 (table 5).
Most specimens from both herbarium and survey collections were G. sarothrae. Gutierrezia microcephala was the second most abundant species collected. Nine other species were represented, with small collection numbers (table 2).
Discussion
Puccinia grindeliae has been present on broom snakeweed in New Mexico and Arizona for at least 88 years and is still present at two sites where P. grindeliae was collected in 1906 and 1907: the Jornada Experimental Range near Las Cruces (specimen 529) and Nara Visa in northeastern New Mexico (specimen 567) (fig. 1b). The rust does not appear to have influenced the range and density of the Gutierrezia spp. populations in the southwest U.S. significantly over the past 100 years. All sites where the rust was found up to 88 years ago are still populated by Gutierrezia species, though at five sites the rust itself could not be found (table 5). Puccinia grindeliae was not found west of the Rio Grande in New Mexico prior to 1990, although it was found in eastern Arizona as early as 1905 at Pinal, in the Santa Catalina Mountains. The pathogen apparently expanded into western New Mexico recently, possibly from Arizona and Mexico due to the prevailing southwesterly winds or human activity. The general increase in the density of Gutierrezia spp. on New Mexico rangelands over the past 100 years (McDaniel et al., 1982; Nadabo et al., 1980) may also have contributed to the establishment of the rust in this area.
Results from the logistic analyses are consistent with the hypothesis that climatic factors affect both the host and pathogen in this interaction. Teliospore germination of the rust is favored by cool, moist conditions (Liddell et al., 1993) and the presence of rust on herbarium specimens was favored by wetter-than-average conditions in the fall of collection. Higher temperatures in the spring quarter also apparently favored the occurrence of rust on herbarium specimens, although the mechanisms responsible for this observation are not clear.
Heitschmidt (1979) found the growth of Gutierrezia spp. in Texas was favored by higher-than-average rainfall in May and lower-than-average daily maximum temperatures in April. Logistic regression analysis of the effect of average daily maximum temperature in April and precipitation in May during the year of collection on the number of herbarium specimens diseased with P. grindeliae showed no significant relationship to rust occurrence. Thus the monthly climatic factors that favor the growth of the host (Heitschmidt, 1979) do not correspond completely with the factors that favor the occurrence of P. grindeliae.
Although the logistic regression analysis implies no cause and effect between independent and dependent variables, the analysis can provide a valuable basis for the formulation of hypotheses. The occurrence of P. grindeliae, based on herbarium specimens, may depend on a moderate level of thermal stress to the host plant in spring, perhaps increasing susceptibility. Growth of Gutierrezia is favored by lower-than-average temperatures in spring (Heitschmidt, 1979); yet rust occurrence on herbarium specimens was correlated with higher mean spring temperatures. The positive correlation between fall precipitation and the occurrence of P. grindeliae appears to directly affect the pathogen, leading to higher levels of teliospore germination and host infection.
We realize there are significant problems in relying on data derived from herbarium specimens to conduct epidemiological and biogeographic studies as plants collected at a site are probably not random samples. Collectors generally select for the best, or representative, specimens and may have avoided diseased plants. These collections were made by hundreds of collectors over the past 100 years, and it is impossible to know exactly what factors influenced the collection of a particular specimen. Older specimens may be in poor condition, providing little helpful data. In addition, the number of positive specimens in any given collection is relatively low. Collection sites may not be chosen precisely and often are not well documented. Repeated collection at a site was rare. There are certainly changes in development and landscaping that lower the likelihood of success when attempting to revisit an established site. However, despite these limitations, herbarium specimens remain the only way to gather information of historical occurrence of many pathogens of noneconomically important plants.
Puccinia grindeliae has been established in native populations of Gutierrezia spp. in New Mexico and Arizona for many years and does not appear to have had a significant impact on those Gutierrezia populations over extended periods of time. Rather than reducing the range and density of Gutierrezia spp., P. grindeliae has spread along with G. sarothrae throughout rangelands in the Southwest over the past 100 years, as may be expected for a biotrophic pathogen at evolutionary stasis with its host. The coevolution of rust pathogens with their hosts has been well established for many systems and it appears that Puccinia grindeliae has been coevolving with Gutierrezia for a considerable time. Given that broom snakeweed is endemic to the southwestern U.S., this result indicates that the long-term effectiveness of P. grindeliae or other endemic species as biological control agents of Gutierrezia spp. in New Mexico and Arizona appears to be limited. Future work to increase the range of P. grindeliae artificially to areas where it is not now present may provide a low level of biological stress on broom snakeweed and enhance the effectiveness of other control and management methods.
References
Brenckle, J.F. (1918). North Dakota Fungi—II. Mycologia 10, 199–221.
Cummins, G.B. (1978). Rust fungi on legumes and composites in North America. University of Arizona Press: Tucson, AZ.
Cummins, G.B. (1979.) Annotated, illustrated, host index of Sonoran Desert rust fungi. Mycotaxon 10, 1–20.
Farr, D.F., Bills, G.F., Chamuris, G.P and Rossman, A.Y. (1989). Fungi on plants and plant products in the United States. APS Press: Minneapolis MN.
Gilbertson, R.L. and McHenry, J. (1969). Check list and host index for Arizona rust fungi. University of Arizona Agricultural Experiment Station Technical Bulletin. 186, 1–40.
Heitschmidt, R.K. (1979). Relative annual broomweed abundance as related to selected climatic factors. Journal of Range Management. 32, 401–403.
Lane, M.A. (1982). Generic limits of Xanthocephalum, Gutierrezia, Amphiachyris, Gymnosperma, Greenella, Thurovia (Compositae: Astereae). Systemic Botany 7, 405–416.
Lane, M.A. (1983). Taxonomy of Xanthocephalum (Compositae: Astereae). Systemic Botany 8, 305–316.
Lane, M.A. (1985). Taxonomy of Gutierrezia (Compositae: Astereae) in North America. Systemic Botany 10, 7–28.
Liddell, C.M., Waddell, C.A. and McEntee, J.P. (1993). Teliospore germination in Puccinia grindeliae, rust of the rangeland weed Gutierrezia sarothrae. Plant Disease 77, 149–152.
McDaniel, K.C., Pieper, R.D. and Donart, G.B. (1982). Grass response following thinning of broom snakeweed. Journal of Range Management 35, 142–145.
McEntee, J.P. and Liddell, C.M. (1991). Geographic distribution of Puccinia grindeliae on Gutierrezia sarothrae in the southwestern USA from 1891 to 1991. Phytopathology 81, 1149 (abstr.).
Nadabo, S., Pieper, R.D., and Beck, R.F. (1980). Growth patterns and biomass relations of Xanthocephalum sarothrae (Pursh) Shinners on sandy soils in southern New Mexico. Journal of Range Management. 33, 394–397.
National Oceanic and Atmospheric Administration, Environmental Data and Information Service, National Climatic Center, Department of Commerce, Arizona and New Mexico Sections (1905–1991).
Peck, C.S. (1879). New species of fungi. Botanical Gazette. 4, 126–128.
SAS Institute Inc. (1989). SAS/STAT User’s Guide, Version 6, Fourth Edition, Volume 2. SAS Institute: Cary, NC.
Solheim, W.G. (1934). Mycoflora Saximontanensis Exsiccata Centum I. University of Wyoming Publication Botany 1, 219–232.
Solheim, W.G. (1940). Mycoflora Saximontanensis Exsiccata Centum III. University of Wyoming Publication 7, 29–42.
Solheim, W.G. (1943). Mycoflora Saximontanensis Exsiccata Centum IV. University of Wyoming Publication 10, 33–46.
Solheim, W.G. (1954). Mycoflora Saximontanensis Exsiccata Centum VI. University of Wyoming Publication 18, 71–82.
Solheim, W.G. and Cummins, G.B. (1957). Mycoflora Saximontanensis Exsiccata Centum VIII. University of Wyoming Publication 21, 56–167.
Solheim, W.G. and Cummins, G.B. (1959). Mycoflora Saximontanensis Exsiccata Centum X. University of Wyoming Publication 23, 38–51.
Solheim, W.G. and Cummins, G.B. (1970). Mycoflora Saximontanensis Exsiccata Centum XIII. University of Wyoming Publication 36, 37–50.
Solheim, W.G. and Cummins, G.B. (1970). Mycoflora Saximontanensis Exsiccata Centum XV. University of Wyoming Publication 36, 69–80.
Solheim, W.G. and Cummins, G.B. (1979). Mycoflora Saximontanensis Exsiccata Centum XVII. Mycotaxon 8, 395–401.
Te Beest, D.O., Yang, X.B. and Cisar, C.R. (1992). The status of biological control of weeds with fungal pathogens. Annual Review of Phytopathology 30, 637–657.
Williams, J.L., ed. (1986). New Mexico in Maps. 2nd Edn. University of New Mexico Press: Albuquerque, NM.
Williams, J.L. and McAllister, P.E., eds. (1981). New Mexico in Maps. University of New Mexico Press: Albuquerque NM.
Yohem, K.H., Cummins, G.B. and Gilbertson, R.L. (1985). Revised list and host index of Arizona rust fungi. Mycotaxon 22, 451–468.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at aces.nmsu.edu.
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
Published and electronically distributed January 1995, Las Cruces, NM.


