Land Application of Treated Industrial Wastewater on a Chihuahuan Desert Shrubland: Water Quality Assessment, Mineral Deposition and Recovery, and Effects on the Vegetation
Bulletin 807
Geno A. Picchioni, John G. Mexal, and Manoj K. Shukla
Agricultural Experiment Station, College of Agricultural, Consumer and Environmental Sciences
Authors: Respectively, Professor, Professor, and Associate Professor, Department of Plant and Environmental Sciences, New Mexico State University ( Print Friendly PDF)
Department of
Plant and Environmental Sciences
Abstract
Land application is a cost-effective wastewater treatment practice that conserves fresh water, recycles nutrients, and mitigates surface water degradation caused by conventional wastewater treatment plant (WWTP) outflows. However, little is known of land application's impacts on arid and semiarid landscapes. We adapted an agrometeorological model for land applying treated, saline–sodic industrial effluent on a Chihuahuan Desert shrubland. Lagoon-treated effluent was applied from 2002–2006 to a 0.4-ha plot to assess the deposition and recovery of applied minerals, changes in soil quality, and impact on the natural vegetation compared to an adjacent 0.4-ha non-irrigated area. Effluent irrigation supplied 26% of the average annual nonstressed evapotranspiration (ET) of the native shrubs [ Larrea tridentata (DC.) Coville and Prosopis glandulosa Torr. var. glandulosa ] and increased both soil stress factors (sodicity, salinity, and pH) and soil fertility (N, P, and K +). After three years, the soil saturation extract electrical conductivity (EC e ) reached 6.1 dS m -1 and Cl – 76 mol c m -3 at 105 cm depth under irrigated L. tridentata . After four years, saturation extract sodium adsorption ratio (SAR e) increased to 35 at 7.5 cm under the irrigated intershrub spaces. There were 27 Mg ha -1 of cumulative ionic land deposits comprising mostly Na +, Cl –, and CaCO 3 equivalent alkalinity. Soil analysis recovered most (>57%) of the mineral deposits except for K + and Na + (8 to 13%). Comparatively small deposits of NO 3 --N, total P, and K + increased soil NO 3 --N, Olsen-P, and soluble K + in the upper 1 m depth, particularly under the shrubs. There was no indication of NO 3 --N leaching below 210 cm, although Cl – movement below a 2 m soil depth revealed the potential for NO 3 --N leaching at higher N loading rates. Herbaceous vegetation in the irrigated intershrub spaces occupied 78% of the land area and produced the highest biomass after 4 yr. With increasing soil sodicity, Lepidium alyssoides A. Gray var. alyssoides became the dominant intershrub space species. Early summer fruit dry weights on terminal branches of the irrigated shrubs were 3 to 14 times more than those on the non-irrigated plot. After 4 yr, approximately 2 Mg of additional aboveground dried biomass (all species combined) had accrued on the irrigated plot compared to the non-irrigated plot, which in turn contained excesses of total Kjeldahl-N (TKN) and Ca 2+ corresponding to around 18% and around 12% of effluent total N and Ca 2+ deposition, respectively. The findings suggest a need for tradeoffs in the management of industrial processing and effluent irrigation. Increasing effluent sodicity may encourage intershrub space biomass by aggressive, apparently natrophilic species such as L. alyssoides , but may also cause shrub injury and a decline in species diversity. Decreasing effluent sodicity may preserve the shrubland community and maintain diversity on the intershrub spaces, but, as shown here, may also reduce total biomass. Thus, sustainable land application in the Chihuahuan Desert must balance vegetation biomass and wastewater attenuation abilities with preservation of the vegetation community. Duplication of this land application model by other Rio Grande communities could mitigate salinization and nutrient loading in the river while reducing wastewater treatment costs. However, further research is needed to determine whether higher effluent application rates cause NO 3 --N leaching and to disclose long-term effects of saline–sodic conditions on Chihuahuan Desert vegetation community structure.
Introduction
Las Cruces, NM, is a rapidly growing Chihuahuan Desert city attracting light industry, general manufacturing, and technology-based companies, creating a need for cost-effective wastewater treatment. Land application is an appropriate wastewater treatment technology for dry, low-fertility sites located near environmentally sensitive waterways (Barton et al., 2005), such as southern New Mexico. The nutrient and water limitations of the Chihuahuan Desert are well recognized (Lajtha and Whitford, 1989; Fisher et al., 1988), and the Rio Grande is at risk from municipal wastewater outflows from conventional WWTPs (Miyamoto et al., 1995; Levings et al., 1998; Anning et al., 2007). Land application catchments could mitigate environmental degradation of the Rio Grande (Doremus, 2008), provide financial benefits to area communities seeking low-cost wastewater treatment, and sustain the natural vegetation.
The Utilities Division of the City of Las Cruces established the 740-ha West Mesa Industrial Park (WMIP) that land applies treated wastewater to native vegetation. The WMIP land application site supports two indigenous perennial shrubs, the evergreen Larrea tridentata (creosote bush) and the winter deciduous Prosopis glandulosa (honey mesquite). In addition, seven indigenous herbaceous species grow within the intershrub spaces. The WMIP includes a secondary WWTP that has received commercial wastewater inflow (influent) from ten industrial tenants. The treated effluent contains plant nutrients that could enhance vegetation growth. However, the effluent is also saline, sodic, and alkaline (Babcock et al., 2009), which poses risk to both soil and vegetation.
Wastewater land applications to agronomic crops, rangelands, forests, recreational areas, and disturbed lands have become common (Ruiz et al., 2006). However, there is limited technical information to guide land managers in semiarid regions, where the fragile vegetation plays a major role in ecosystem structure and function (McAuliffe et al., 2007; Cross and Schlesinger, 1999), and where saline–sodic water could have significant impacts on the soil and natural vegetation. Several New Mexico towns have established land application sites, and others have expressed an interest in land application for wastewater treatment. Presently, there are no environmental impact data, such as groundwater contamination, salt and nutrient deposition, soil salinization, and vegetation stress, that could aid in the design and operation of sustainable wastewater land application practices in the Chihuahuan Desert.
Improved management tools could increase sustainability of land application systems (Bastian, 2005). In a 4-yr land application study in semiarid Wyoming (Ganjegunte et al., 2008), irrigation with saline–sodic wastewater did not account for vegetation water demands, and the authors posed a need for management strategies to minimize adverse effects of salinity and sodicity. In the present study, wastewater application frequency and amount were not arbitrary but were based on the average ET of the native shrubs, L. tridentata and P. glandulosa, by adapting an agrometeorological (crop coefficient) model for the semiarid region (Sammis et al., 1985). This irrigation scheduling procedure can be replicated for other Chihuahuan Desert communities (Ruiz et al., 2006). While we have acknowledged the limitations of this method for sparse desert vegetation, it represented a systematic rationale for irrigation scheduling and amount, and a first approximation for Chihuahuan Desert vegetation in situ (Picchioni et al., 2012a).
Chihuahuan Desert field studies have reported increased growth of L. tridentata in response to water and N (Lajtha and Whitford, 1989; Ettershank et al., 1978; Fisher et al., 1988). Similar observations apply for short-term studies of young P. glandulosa seedlings in controlled environments (Jarrell and Virginia, 1990a; Maestre and Reynolds, 2006; Causin et al., 2004). However, lack of field data on uptake and recovery of wastewater minerals by native vegetation presents biological, agrotechnical, and environmental impediments toward establishing sustainable land application systems. Plant nutrient uptake is regarded as a key biogeochemical process affecting the natural wastewater attenuating ability of the land (Miller et al., 2008; Bastian, 2005). In New Mexico, discharging contaminants to land requires a pollution prevention permit from the New Mexico Environment Department's Ground Water Quality Bureau (NMED-GWQB) to ensure that the contaminants are being utilized (or destroyed) and not simply moved from one place to another (Faris, 2009). Thus, addressing the nutrient uptake scientific deficit is essential to developing land application systems in New Mexico.
Field salt tolerance data on L. tridentata and P. glandulosa that could help predict salinity effects of land application at the WMIP are lacking. Other than a limited observation supporting a high level of salt tolerance of P. glandulosa under field conditions (Jarrell and Virginia, 1984), only short-term controlled environment studies are available. For example, P. glandulosa seedlings withstood electrical conductivity of a soil saturation extract (EC e) that exceeded 20 dS m -1 (Jarrell and Virginia, 1990a), and an EC of culture solutions exceeding 9 dS m -1 (Felker et al., 1981). Larrea tridentata had no growth reduction with a culture solution EC of up to 7.5 dS m -1 (Al-Jibury, 1972). This information supports the possibility that these shrubs would tolerate increased soil salinity caused by effluent land application at the WMIP.
We found no data pertaining to irrigation, fertilization, and salinity responses by the intershrub herbaceous plant species at the WMIP. Huenneke et al. (2001) reported that annual and perennial forbs are of limited importance to Chihuahuan Desert community biomass. However, Gutierrez and Whitford (1987) found that soil additions of water and N increased biomass and density of several Chihuahuan Desert annual species, which suggests that WMIP herbaceous species biomass could be enhanced by land-applied effluent.
Given the widely accepted desert ecological principle of nutrient and water co-limitation (Ettershank et al., 1978; Sharifi et al., 1990; Fisher et al., 1988; Gutierrez and Whitford, 1987; Schlesinger et al., 1996; Mackay et al., 1987) and the limited data from short-term, controlled environment studies supporting salt tolerance of Chihuahuan Desert shrubs, we hypothesized that the Chihuahuan Desert vegetation at the WMIP would respond opportunistically to the nutrient-containing treated effluent in the presence of increased soil salinity, sodicity, and pH. In view of the lack of data on the impact of wastewater application on soil properties and natural vegetation in southern New Mexico, the objectives of this 4-yr study were as follows: i) apply an agrometeorological irrigation scheduling model to effluent application, ii) assess WWTP performance, iii) measure deposition and soil recovery of effluent components, iv) evaluate soil quality changes, v) monitor vegetation growth, and vi) estimate vegetation mineral recovery in response to treated wastewater application on a Chihuahuan Desert shrubland. This bulletin represents a comprehensive assessment of the 4-yr study with more condensed reports available in Picchioni et al. (2012a, 2012b).
Materials and Methods
Study site, WWTP, and land application system
The study was conducted between 2002 and 2006 at the WMIP (106 o54' W, 32 o16' N; elevation 1,190 m), 10 km west of Las Cruces, New Mexico. The mean annual temperature is 16 oC and the mean annual rainfall of 22 cm (mostly between July and September) is 9% of the mean annual pan evaporation of 239 cm in Las Cruces (New Mexico Climate Center, 2011). The natural vegetation includes two perennial shrubs, evergreen L. tridentata and deciduous P. glandulosa, and several herbaceous species in the intershrub spaces. The soil is an alkaline Bluepoint sand (calcareous, mixed, thermic, Typic Torripsamment) with CaCO 3 deposits at a depth of 0.5 m to 1.5 m and water table depth at approximately 100 m (Bulloch and Neher, 1980; Gile et al., 1981). Particle size distribution, bulk density, saturation percentage, and soil moisture retention showed low variability across the site and through depths, and are reported elsewhere (Adhikari et al., 2011; Babcock et al., 2009).
The Utilities Division of the City of Las Cruces is authorized by NMED-GWQB to land apply treated effluent originating from untreated wastewater influent produced by ten industrial tenants. The WMIP includes a secondary WWTP that treats up to 1,500 m 3 d -1 of influent, and 36 ha of Chihuahuan Desert shrubland for application of treated effluent. The untreated influent is passed through two synthetically-lined, aerated lagoons, each equipped with a fixed floor fine bubble diffuser system. The first lagoon is a complete mix process with a volume of 4,500 m 3, retention time of 6 d, and a design influent flow of 750 m 3 d -1. The second partial mix lagoon of 9,000 m 3 settles the solids. The final stage of the WWTP is a holding and equalization pond with a capacity of 4,000 m 3.
Flow meters monitored influent and effluent volumes. The treated effluent was applied through an overhead irrigation system by fixed-head sprinklers (Senninger #3012, 1-3/4, Senninger Irrigation Co., Inc., Clermont, FL) installed on a 12 m x 12 m grid atop underground water lines with sprinkler height at ≈0.6 m above ground. The designed pressure of 0.3 MPa provided a flow rate of 0.3 L sec -1 and a precipitation rate of 0.74 cm h -1. All WWTP and irrigation system operations were performed by the City of Las Cruces.
Experimental plots and irrigation scheduling
An unblocked experimental design was nEC essary for working within system constraints. Within the 36-ha land application area, two adjacent 0.4-ha experimental plots were measured and marked in fall 2001. This plot size was previously determined to be adequate for characterizing Chihuahuan Desert biomass and aboveground net primary production (ANPP) (Huenneke et al., 2001). For the ensuing 4-yr study, sprinkler heads were capped on the non-irrigated plot, which received only rainfall, while the irrigated plot received effluent in addition to rainfall. The plots were separated in their lengthwise dimension by 24 m, with each plot measuring 24 m wide x 150 m long. Weekly treated effluent applications to the irrigated plot began on February 5, 2002. Irrigation scheduling was based on average shrub ET (Ruiz et al., 2006; Babcock et al., 2009). During irrigation, no surface runoff was observed, which was consistent with the low antEC edent soil moisture content. In summer 2004, irrigation scheduling workshops for Las Cruces and El Paso City Utilities employees were led by project participant A. Ruiz, assisted by other project personnel. Starting in January 2005, the irrigation scheduling technique was adopted by the City of Las Cruces for land application at the WMIP.
We defined our populations as the individual plots to which the treatments were applied. Soil and vegetation samples within each population were considered replications to estimate variability of the populations. Thus, comparisons are limited to differences among the plots within our study area (Wester, 1992).
Influent and effluent sampling and chemical analysis, and effluent mineral deposition
The influent and treated effluent were analyzed in quadruplicate grab samples collected in plastic bottles, following the procedures of United States Environmental Protection Agency (USEPA, 1982). Unless noted otherwise, the quadruplicate samples were collected at monthly intervals in 2002 and at approximately quarterly intervals thereafter. The EC was obtained, and standard methods from the American Public Health Association (APHA) et al. (1998) were used to determine dissolved plus particulate fractions (total digest) for total Na +, Ca 2+, Mg 2+, and K + (200.7); total Cl – and NO 2 – + NO 3 –-N (300.0); total alkalinity as CaCO 3 equivalents (2320B); total Kjeldahl N (TKN) (4500-NH 3C); total NH 4 +-N (4500-NH 3H); total P (365.1/4500-PF); and chemical oxygen demand (COD) (5220D). The SAR was calculated as Na +/(Ca 2+ + Mg 2+) 1/2 (ion concentrations in mmol L -1). Biological oxygen demand (BOD) was determined by standard method 5210B described in APHA et al. (1992). Total suspended solids (TSS) were measured by method 160.2 (USEPA, 1979). Heavy metal determinations in the total digest followed standard methods in APHA et al. (1998) as follows: Al, Cu, Cr, Mo, Ni, Se, and Zn (200.7); As (206.2); Cd (213.2); Pb (239.2); Hg (245.1); and Ag (272.2).
Annual deposits to the 0.4-ha irrigated plot were determined for TSS; TKN-N; NO 2 –/NO 3 –-N; total N, P, K +, Ca 2+, Mg 2+, Na –, Cl –; and total alkalinity. Annual deposition was calculated as the product of annual effluent land application volume (m 3) by the average annual effluent component concentration in mg L -1. Deposition values are reported in kg ha -1.
Soil sampling, analysis, and recovery of effluent-applied minerals
A preliminary soil analysis was made on the non-irrigated plot in October 2001, four months prior to the start of land application to the adjacent irrigated plot. Results were similar to those obtained from the non-irrigated plot in December 2002, or after one growing season of land application. Data for the preliminary soil analysis are not presented.
During the land application period of 2002–2005, the soil was sampled every December. Bulked core samples were obtained with a 10-cm wide x 30-cm long metal auger at depths of 0–15 cm, 15–30 cm, and 30–60 cm, then in additional 30-cm increments to a final depth of 210 cm (eight total depths). We obtained triplicate samples under each of the three ground types of intershrub space and the drip lines of L. tridentata and P. glandulosa. Sampling sites were randomly determined within each third of the plot length to ensure an even soil sampling distribution throughout the plots. In addition to the annual December soil samples, single soil samples were collected between August 2004 and August 2005 at 0–30 cm and 60–90 cm depths. This period coincided with relatively high effluent Na + deposition, and results are discussed in the text where appropriate. Further soil sampling details are in Babcock et al. (2009).
The soil samples were air-dried, thoroughly mixed, and passed through a 2-mm sieve, and a representative subsample was used for chemical analysis. It was assumed that the overall analytical values obtained from the subsample represented the average of the incremental depth as presented in the figures. The soil saturation extract was prepared (Rhoades, 1996) and the EC e and pH determined. The concentrations of Na +, Ca 2+, and Mg 2+ in the saturation extract (mol c m -3), and of K + in a 1:5 soil:water extract (reported in mg kg -1 dry weight), were determined using an inductively-coupled plasma emission spectrometer (ICP-ES) (Optima 4300, Perkin Elmer, Waltham, MA). The saturation extract sodium adsorption ratio (SAR e) was calculated as described previously for the water samples. Soil NO 3 --N concentration in a 1:5 soil:water extract (mg kg -1 dry weight), NH 4 +-N in the saturation extract (mg L -1), and saturation extract Cl – (mol c m -3) were determined by colorimetric procedures of Mulvaney (1996) for NO 3 --N and NH 4 +-N, and of Frankenberger et al. (1996) for Cl –, both by an autoanalyzer (AAII, Technicon Instruments, Tarrytown, NY). The NaHCO 3-extractable soil P (mg kg -1 dry weight) was obtained by the method of Olsen et al. (1954) using a spectrophotometer (Spectronic 20B, Thermo Fisher Scientific, Waltham, MA). Soil organic matter was determined using the Walkley-Black method (Nelson and Sommers, 1996). Soil TKN (mg kg -1 dry weight) was determined by the method of Bremner (1996) using the autoanalyzer. Total P in soil solids (mg kg -1 dry weight) was analyzed by ICP-ES following the microwave digestion procedures (EPA method 3051) that are described in USEPA (1997).
Soil analytical data are presented as the mean ± SE of triplicate determinations per ground type, irrigation treatment, and year, and at the average (midpoint) sampled depth (e.g., 7.5 cm average depth for the 0–15 cm bulk core, and so on for the remaining cores to 180–210 cm). Two-sample t-tests were performed to analyze differences in soil chemical properties between the non-irrigated and irrigated plots at P ≤ 0.05 within year, ground type, and soil depth. Saturation extract total dissolved cations and anions (TDC and TDA, respectively) were computed considering the dominant electrolytes of Cl –, Na +, Ca 2+, Mg 2+, and K + (K + converted to mol c m -3 using soil saturation percentage), and only in the 60–90 cm soil depth, which was at or near maximum salinity on the irrigated plot. Soil NO 3 --N and NH 4 +-N were inconsequential to the balances and were excluded. Soil saturation extract HCO 3 –, CO 3 2–, and SO 4 2– were not assessed because Cl – alone balanced an average of 113% of the TDC pool across the irrigated ground types, and for all years.
Total contents (kg ha -1) of soil TKN, NO 3 --N, total P, and soluble K +, Ca 2+, Mg 2+, Na +, and Cl – were obtained by summing the products of concentration by dry soil weight through all depths (determined using bulk density), after accounting for land area under the ground types. Of the total land area in both non-irrigated and irrigated plots, intershrub space occupied 78%, L. tridentata 9%, and P. glandulosa 13%. For soluble ions in the soil saturation extract (Ca 2+, Mg 2+, Na +, and Cl –), equivalent weight and saturation percentage were included in the conversion from concentration to content.
Total soil mineral content data are presented only for December 2005, and as the mean of triplicate ground type sampling site determinations. The absolute differences in December 2005 soil mineral contents between the non-irrigated plot and the irrigated plot (kg ha -1) were expressed as percentages of cumulative effluent mineral deposition through December 2005 (kg ha -1), reflecting recovery of applied effluent minerals throughout the 210-cm depth of soil at the end of the land application period.
Vegetation sampling targets
Destructive vegetation sampling was restricted to aboveground tissues and made at approximately quarterly intervals (late winter, early summer, and early fall) to coincide with seasonal biomass production peaks. The experimental plots were divided into equal parts to ensure that multiple sample sites for different vegetation were evenly spaced. A similar plot-dividing procedure for Chihuahuan Desert vegetation sampling was reported by Miller and Huenneke (2000a), Molinar et al. (2002), Lajtha (1987), and Ettershank et al. (1978).
Intershrub space herbaceous vegetation sampling
Seven indigenous annual and perennial herbaceous plant species were identified on the intershrub spaces. Six minor species made up less than 0.05% of total biomass and were ignored. The summer annuals included Boerhavia spicata Choisy (creeping spiderling), Pectis papposa Harv. E.A. Gray (chinchweed), and Tidestromia lanuginosa (Nutt.) Standl. (wooly tidestromia). The perennials included Bahia absinthifolia Benth. (desert daisy), Croton pottsii (Klotzsch) Müll. Arg. (leatherweed), and Gutierrezia microcephala (DC.) A. Gray (threadleaf snakeweed). For simplicity, we refer to G. microcephala as an herbaceous species, although the functional classification is shrub or subshrub (Natural Resources Conservation Service [NRCS], 2012a). The seventh herbaceous species, Lepidium alyssoides A. Gray var. alyssoides (mesa pepperwort), is reported to grow as a biennial or perennial (NRCS, 2012a). In our relatively warm climate, this species appeared to behave as a perennial.
Sampling dates for the intershrub space herbaceous vegetation were October 4, 2002; October 21, 2004; and October 26, 2005, before autumn senescence and the first fall frost. The length of each plot was divided into 10 equal sections of around 400 m 2; within each section, a 1-m 2 wooden frame was randomly positioned. Within the frame, all of the aboveground (shoot) tissues were harvested at ground level and the dry weights of each species were determined after drying at 60 oC. Dry weight was expressed per 1-m 2 frame and per ha basis after accounting for the intershrub space land cover of 78%.
Dried tissues were subjected to mineral analysis as described in the Vegetation mineral analysis section. To reduce analytical costs, mineral determinations were made based on the combined tissues of all species. Mineral concentrations are not presented, although they are briefly discussed in the text where appropriate. Shoot mineral content was calculated as the product of total dry matter and mineral concentration, and expressed in kg ha -1.
Two-sample t-tests (P ≤ 0.05) were performed within each year to compare the average total dry matter and mineral contents between the non-irrigated and irrigated plots. Summation of 3-yr cumulative differences in total shoot dry matter and mineral contents (irrigated minus non-irrigated plot) were obtained. Cumulative differences in mineral content are presented as percentages of the cumulative 3-yr effluent mineral deposition (2002, 2004, and 2005).
Shrub whole canopy sampling
On each 0.4-ha plot, L. tridentata canopies covered 9% (356–362 m 2) and P. glandulosa canopies covered 13% (523–531 m 2) of the total ground area. Plot shrub surveys were conducted during the growing seasons to determine total number of shrubs and canopy diameters of every shrub on both non-irrigated and irrigated plots. Diameter of each shrub canopy was defined as an average of the two maximum canopy widths in the cardinal directions (Miller and Huenneke, 2000a).
Shrub canopies were harvested beginning February 5, 2002; March 15, 2004; and March 15, 2005, which is the late dormant season in local conditions. Each harvest required 7 to 10 d. Because of constraints on the number of shrubs that could be destructively harvested, linear regression analysis was applied to the approximate range of natural shrub size on the plots, similar to Hughes et al. (2006) and Huenneke et al. (2001). An exception was the 2002 P. glandulosa harvest, which was limited to a relatively small shrub size range compared to the plot population range (those data were excluded from regression analyses). The two 150-m-long plots were divided into five 30-m-long sections running in the lengthwise dimension. We then applied a stratified sampling approach by dividing the natural range of shrub canopy diameter into five discrete intervals and randomly selecting a size interval for each plot section (n = 5 shrubs per harvest). Shrubs were harvested by cutting the main branches to ground level where the average basal branch diameter among five sampled shrubs ranged from 1.5 to 3.3 cm for L. tridentata and from 2.7 to 4.5 cm for P. glandulosa. Depending on shrub size, each harvest included 4 to 44 L. tridentata main branches per shrub and 1 to 32 P. glandulosa main branches per shrub.
To evaluate whether the destructively harvested shrubs of 2004 and 2005 were representative of the broader population of shrubs, at the close of the study in 2006, Pearson's chi-square analysis was performed on the observed percentile of the harvested shrub average canopy diameters. The observed percentiles were calculated from among all shrubs in the study for each species and irrigation treatment combination. The chi-square test statistic along with the P values were calculated in Microsoft Excel Version 12.1.0 (Microsoft Corp., Redmond, WA). Expected percentiles for each of the 10 plants in each of the treatment by species combinations were simply i/11, where i = 1...10. A significant P value indicated non-uniformity of sampled shrubs compared to the plot population. All but one of the treatment by species combinations provided P values ranging from 0.6091 to 0.8790, and thus there was no significant evidence that harvested shrubs were non-representative of entire populations. A significant P value for non-irrigated P. glandulosa (P = 0.0257) may have been a reflection of limited shrub availability and may have also been associated with inherently high shrub-to-shrub variability of this Chihuahuan Desert species (Huenneke et al., 2002). A low correlation between shrub size and biomass for non-irrigated P. glandulosa in 2005 is additional evidence for this variability.
The 2002 harvest was made at the onset of land application (February 5) to determine initial shrub biomass and included five L. tridentata shrubs and six P. glandulosa shrubs immediately outside the study area. In 2004 and 2005, five shrubs per species were harvested within each of the plots. For L. tridentata, the entire canopy was harvested and weighed, and a subsample of four main branches was randomly selected and stored at 5 oC to await processing. For P. glandulosa, we harvested the entire canopy of shrubs less than 3 m in diameter, one-half of the canopy of shrubs 3 to 5 m in diameter, and one-fourth of the canopy of shrubs wider than 5 m in diameter (specific halves or quarters randomly determined). The P. glandulosa canopy tissues were weighed, and subsamples were collected as described for L. tridentata.
The L. tridentata canopy subsamples were fractionated into leaves, twigs (wood <0.5 cm in diameter), and branches (all wood >0.5 cm in diameter), and the fresh weights were recorded. The leafless P. glandulosa subsample branches were fractionated into twigs and branches as described for L. tridentata, and the fresh weights were determined. All branch subsample tissues were dried at 60 oC and weighed. The fresh weight:dry weight ratio of the combined subsample tissues was used to estimate total dry weight of the harvested material remaining in the field, which was then added to the subsample dry weight to determine total canopy dry weight. For the partial P. glandulosa canopy harvests, the dry weights were adjusted to a full canopy size for presentation purposes. All dried tissues were saved for mineral analysis.
Yearly linear regression equations were generated for the relationship between shrub projected canopy area (PCA) and total aboveground shrub biomass. Regression slope and elevation comparisons between the non-irrigated and irrigated plots were then performed using F-tests (Snedecor and Cochran [1989] on Statistix 9 [Analytical Software, Tallahassee, FL]). The coefficients of determination (R 2) are reported with the regression data. For each shrub species, the total aboveground biomass in the 0.4-ha plots was estimated for the March 2005 shrub harvest by applying the PCA of each shrub that was obtained from the plot shrub survey (including 2004 and 2005 harvested shrubs) to the regression equations, and summing across all shrubs on the plot (Miller and Huenneke, 2000a). The differences in L. tridentata and P. glandulosa aboveground biomass between the non-irrigated and irrigated plot at March 2005 were calculated by per-ha basis and are presented as a shrub whole canopy biomass total for both species combined.
To determine possible influences of shrub size on shrub mineral concentrations, we performed t-tests (Statistix 9) for significance of slope for the relationship between shrub PCA and mineral concentration. For only 10 of the 154 combinations of shrub species x shrub organ x irrigation treatment x mineral x year was there a significant slope (P ≤ 0.05). In the minority of cases involving a significant P, we multiplied each shrub's organ mineral concentration by its individual organ dry weight in order to determine total mineral weight per organ per shrub. In the majority of cases (non-significant P), we multiplied the average mineral concentration of an organ across all shrubs per species, treatment, and harvest by the individual shrub organ dry weight to estimate total mineral weight per organ per shrub. Mineral weights were then determined by summation of the products of mineral concentration by subsample organ dry weight and adjusting to an entire shrub basis. Linear regression equations were developed for the relationship between mineral weight per shrub and shrub PCA. Slope and elevation comparisons between the non-irrigated and irrigated plot were performed, and regression equations are presented only for Na + and Cl – weight per shrub, since Na + and Cl – were dominant ions in the effluent and soil analysis, and due to lack of TKN, P, K +, Ca 2+, and Mg 2+ concentration differences in the non-irrigated and irrigated plot shrub canopy tissues.
For the March 2005 sample date only, we calculated total aboveground TKN, P, K +, Ca 2+, Mg 2+, Na +, and Cl – content for both L. tridentata and P. glandulosa in the 0.4-ha plots by the same procedure used for plot biomass estimation described previously, i.e., integrating regression equations with the plot shrub survey. We then expressed the excesses in mineral content on the irrigated plot over the non-irrigated plot on a unit ha basis. The differences were combined for both shrub species and expressed as a percentage of cumulative land-applied mineral deposition during 2002, 2003, and 2004.
Shrub terminal branch sampling
Within both the non-irrigated and irrigated plots, one shrub per species was randomly selected in each of the five plot sections established for the shrub canopy harvests. Shrubs were selected so that their average canopy diameter was within one SD of the plot population determined in the plot shrub survey. Terminal branches were harvested during early summer 2002 (July 1), 2004 (July 14), and 2006 (June 22), and during fall 2002 (October 1) and 2004 (October 5). Results of the fall samples are limited to the text. The canopies of the selected shrubs were divided into quarters at the cardinal directions. A single branch at the outer periphery of the canopy measuring 30 cm in length with basal branch diameter of 0.7 cm was harvested at each canopy coordinate. The branches were separated into leaves, wood, and, when applicable, fruit. Total area of fresh leaves from each individual terminal branch harvested in July 2002 and July 2004 ( P. glandulosa rachii included) was determined using a portable area meter (LI-COR 3000; LI-COR, Lincoln, NE).
At the July 2004 sampling date, freshly harvested leaves from the irrigated plot were subjected to a leaf washing procedure to determine possible surface mineral residues from the overhead irrigation. From each of the five shrubs, the four terminal branch leaf samples were pooled, and 1.5-g subsamples were divided into two equal fractions. One of the fractions was washed in 50 mL deionized water for 3 min with gentle mixing, while the other fraction was unwashed. The paired samples from five shrubs per species were dried at 60°C, and mineral analysis was performed. Mineral concentrations in the washed sample were expressed as a percentage of mineral concentrations in the unwashed sample, and the results are briefly discussed.
Dry weights of the individual and combined terminal branch components were obtained, and the samples were retained for mineral analysis. The four branches per shrub were pooled to determine a shrub average for wood, leaf, and, if applicable, fruit dry weight per branch, total dry weight per branch, and mineral concentrations. Early summer terminal branch tissue Na + and Cl – concentrations are included in the presentation due to significant effects of effluent irrigation. The mineral weight per branch was determined by summing individual products of average mineral concentration per organ and the average shrub organ dry weight. The non-irrigated and irrigated plot shrub species averages comprised five individual shrubs at each sample date, with each shrub consisting of four pooled branches (total of 20 terminal branches per observation). Two-sample t-tests (P ≤ 0.05) were performed to compare dry weight and mineral concentrations of the terminal branch components, and total dry weight and total mineral content per branch between the non-irrigated and irrigated plots.
At the July 2004 sample date, we counted the total number of terminal branches on each of the five non-irrigated and irrigated shrubs. There were no significant differences in terminal branch counts per shrub between the non-irrigated and irrigated plots (P ≤ 0.05), with shrub averages of 95 ± 11 for L. tridentata and 123 ± 20 for P. glandulosa. The per-branch biomass and mineral weight data were converted to a per ha basis (Ettershank et al., 1978) for the June 2006 sample date only, by which time there were significant fruit biomass enhancements on the terminal branches of the irrigated shrubs. At no other terminal branch sampling date was there such an enhancement of total branch dry weight for both shrub species; therefore, terminal branch dry weight data per plot and land area are not presented for 2002 and 2004. Because we sampled shrubs within 1 SD of the average canopy diameter of the plot populations (average of 65% of the population number in each plot x species combination), the data did not account for small and large shrub individuals outside of 1 SD, and thus our estimates apply only to "average" shrub conditions and not the entire plot populations.
We estimated the terminal branch mineral recovery per ha in combined L. tridentata and P. glandulosa (irrigated plot minus non-irrigated plot) at the June 2006 terminal branch sample date and expressed the recovery as a percentage of the rEC ent 2005 annual mineral deposition. We discounted mineral deposition in 2006 due to a lack of effluent supply in that season. Thus, soil and mineral deposition analyses completed by December 2005 largely reflected the soil conditions at the June 2006 terminal branch sampling date.
Vegetation mineral analysis
The dried herbaceous shoot tissues and dried leaves and fruit of the shrubs were ground in a Wiley mill to pass a 40-mesh screen. The woody shrub fractions were reduced into small fragments by a motorized chipper, then ground in the Wiley mill. The ground tissues were thoroughly mixed, and 0.25- to 0.5-g subsamples were withdrawn for mineral extractions. The TKN was extracted by acid block digestion with analysis on the autoanalyzer by the methods of Gavlak et al. (1994). A second subsample was subjected to microwave digestion (Jones et al., 1991) for determination of P, K +, Ca 2+, Mg 2+, and Na + by ICP-ES. From a third subsample, Cl – was extracted in acetic acid (Gavlak et al., 1994) and determined on the autoanalyzer by standard method 4500-Cl-E (APHA et al., 1995). Plant mineral concentration data are expressed as a percentage of the subsample dry weight.
Results and Discussion
Influent supply, influent and effluent analysis, and WWTP performance
The WMIP well water had an EC of 0.73 dS m -1, pH of 7.85, SAR of 1.5, CaCO 3 equivalent alkalinity of 2.3 mol c m -3, and Cl – of 2.5 mol c m -3. The WWTP received a total of 399,677 m 3 untreated influent through the 4-yr study, with annual receiving flows varying between 64,472 and 144,103 m 3. Tenant discharge volume (and thus effluent availability) was the highest in 2004 (see Effluent irrigation section). Of the total influent, a cheese processing plant provided 46% and a specialty wire fabricator provided 22%, with minor contributions from the eight remaining tenants: two metal fabrication companies, an electrical controller manufacturer, a convenience store with truck stop, a food and beverage confectioner, a wholesale plant nursery, a juvenile detention center, and a flatbed truck transportation firm.
Influent and effluent heavy metal concentrations were generally at or below detection limits. The average 2002 influent EC was the lowest of all years at 1.7 dS m -1 (Table 1). Thereafter, average annual influent EC increased by between 0.1 and 0.6 dS m -1 each year, and reached a maximum of 2.8 dS m -1 in 2005. The 4-yr average effluent EC (3.6 dS m -1) was 1.6 times higher than the 4-yr average influent EC (2.2 dS m -1). Likewise, the TDC, TDA, Na +, K +, Cl –, and CaCO 3 equivalent concentrations in the effluent averaged 1.2 to 2.3 times higher than in the influent (Table 1). These differences reflected evaporation from the lagoons and holding pond and possible salt accumulation over time. Influent and effluent Mg 2+ concentrations (Table 1) were similar and relatively low. In 2002, the effluent Ca 2+ concentration was higher than the influent Ca 2+ concentration (Table 1). However, from 2003–2005, the effluent Ca 2+ concentration averaged five times lower than the influent Ca 2+ concentration because of Ca 2+ precipitation and sedimentation in the second lagoon (see following discussion).
Table 1. Annual averages for ionic properties of the untreated wastewater influent produced by the Las Cruces West Mesa Industrial Park (Infl.) and the treated wastewater effluent (Effl.) used for plot irrigation from 2002 to 2005. In 2002, the influent and effluent waters were sampled 12 times (monthly) with exceptions noted in the footnote below; in 2003 and 2004, the waters were sampled 5 times per year at intervals spaced an average of 2.4 months; in 2005, the waters were sampled 4 times at 3-month intervals. The Na +, Ca 2+, Mg 2+, K +, and Cl – analyses included dissolved plus particulate fractions (analysis on total digest). The standard error of the mean (SE) is reported as an average across all four years.
| Concentration (mol c m -3) | ||||||||||||||||||||
|
EC |
SAR b | TDC c | TDA d | Na + | Ca 2+ | Mg 2+ | K + | Cl - | Alkalinity e | |||||||||||
|
Year |
Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. |
| 2002 |
1.7 |
4.3 | 13.2 f | 14.3 f | 25.1 f | 36.2 f | 23.5 f | 36.6 f | 12.8 | 30.3 | 3.5 f | 5.2 f | 1.0 f | 2.0 f | 1.0 | 1.8 | 8.0 | 32.0 | 9.2 | 6.9 |
| 2003 | 2.1 | 3.3 | 10.4 | 26.1 | 24.5 | 33.5 | 21.4 | 31.2 | 17.2 | 28.3 | 4.4 | 1.3 | 1.0 | 1.1 | 1.9 | 2.9 | 5.0 | 9.2 | 16.5 | 22.0 |
| 2004 | 2.2 | 2.9 | 12.1 | 28.1 | 26.9 | 30.3 | 23.9 | 26.2 | 19.8 | 26.1 | 4.4 | 0.8 | 0.9 | 0.9 | 1.8 | 2.5 | 5.0 | 6.9 | 18.9 | 19.3 |
| 2005 | 2.8 | 4.0 | 11.3 | 42.5 | 31.6 | 44.9 | 25.8 | 39.2 | 21.8 | 39.3 | 5.9 | 0.6 | 1.3 | 1.2 | 2.5 | 3.8 | 6.8 | 9.3 | 19.0 | 29.9 |
| SE | 0.4 | 0.3 | 2.4 | 3.0 | 4.8 | 3.8 | 4.0 | 3.3 | 3.3 | 2.8 | 0.5 | 0.3 | 0.1 | 0.2 | 0.4 | 0.3 | 0.7 | 1.4 | 2.9 |
2.0 |
| aElectrical conductivity. bSodium adsorption ratio calculated as follows: Na +/(Ca 2+ + Mg 2+)1/2; all ion concentrations in mmol L -1. cTotal dissolved cations calculated from individual ionic means (Na +, Ca 2+, Mg 2+, and K +). dTotal dissolved anions calculated from individual ionic means (Cl – and alkalinity). Slight deficits in TDA relative to TDC may be attributed to exclusion of SO 4 2- from analyses. eExpressed as CaCO 3 equivalents. fIn 2002, determinations made only for the months of October, November, and December. |
||||||||||||||||||||
Influent SAR averaged between 10 and 13 (Table 1). From 2003–2005, effluent SAR ranged between 26 and 43, a very high irrigation water sodicity hazard (United States Salinity Laboratory Staff, 1954). The high effluent SAR was mostly related to the declines in effluent Ca 2+ concentration rather than to changes in Na + or Mg 2+. Sodium made up 51% of influent TDC in 2002 and 71% of influent TDC thereafter, as the influent Na + concentration increased while influent Ca 2+ and Mg 2+ increased less markedly or not at all. In the effluent, Na + represented an average of 86% of TDC throughout the study.
In 2002, the influent Cl – concentration ranged from 5 to 19 mol c m -3 while the effluent Cl – concentration reached as high as 50 mol c m -3 to account for 87% of effluent TDA (Table 1). In December 2002, the City of Las Cruces advised the tenants to limit Cl – discharge concentration to 7 mol c m -3 (250 mg L -1) for compliance with the NMED-GWQB permit. That action was associated with the increase in influent EC noted previously, increase in influent and effluent total alkalinity, and decrease in influent and effluent Cl – (Table 1). Whereas total alkalinity made up an average of 29% of influent and effluent TDA in 2002, the 2003–2005 anion composition of both influent and effluent was alkalinity-dominated (75% of TDA). After several queries to the City of Las Cruces, we were unable to obtain reliable information on how the tenants modified their Cl – discharge compliance processing methods that increased total salinity and alkalinity of the influent, other than our speculation that there was an increase in the use of alternative cleaning compounds such as alkaline detergents and caustic sodas involved in cheese processing (Liu and Haynes, 2010; Britz et al., 2006) in place of chlorinating disinfectants.
Compliance with the NMED-GWQB permit regulation to limit Cl – discharge may have negative ramifications for lagoon management, soil, and vegetation. The beginning of 2003 irrigation marked the beginning of high influent alkalinity. The differences between high average annual influent and low average annual effluent Ca 2+ concentrations in 2003 and 2004 were 3.1 and 3.6 mol c m -1 (1.6 and 1.8 mmol L -1), respectively (Table 1). The differences between high average annual influent and low average annual effluent TSS concentrations in 2003 and 2004 were 619.8 and 728.6 mg L -1, respectively (Table 2). The total influent flows to the WWTP were 109,485 m 3 in 2003 and 144,103 m 3 in 2004. Multiplying the average annual Ca 2+ concentration differences (molar basis) by the annual influent flows and summing the products, there were 17.2 Mg of Ca 2+ that theoretically fell to the bottom of the second (settling) lagoon during 2003 and 2004. Likewise, multiplying the annual TSS concentration differences by the annual flows and summing those products, there were 172.9 Mg of TSS that were theoretically deposited in the lagoon in 2003 and 2004, giving a projected Ca 2+ concentration in the lagoon sediment of 9.95%. The City of Las Cruces performed a total Ca 2+ analysis of the second lagoon sludge in October 2004 using method 200.7 described in USEPA (1994). The average Ca 2+ concentration in triplicate digests was 9.97% of sludge dry weight (CV = 18%), which was identical to our mass balance calculation. The corroborating data confirm that the decreases in effluent Ca 2+ concentrations relative to influent Ca 2+ concentrations from 2003–2005 could only be attributed to Ca 2+ precipitation and probably as insoluble calcite. Adding the mass contribution by CO 3 2- (the likely co-precipitating anion), as much as 25% of sludge formation could have resulted from calcite sedimentation. Not only would the sludge need to be removed from the lagoon but the increased soil sodicity hazard from treated effluent irrigation (resulting from Ca 2+ precipitation and reduced Ca 2+ proportion in the water) could result in damaging effects to the soil and vegetation (see subsequent sections on soil and vegetation analyses).
The average annual influent TKN concentration doubled between 2002 and 2003, decreased slightly in 2004, and rose sharply once again in 2005 (Table 2). The rising influent TKN concentrations after 2002 may have reflected additions of organic-N compounds in cheese whey (Ghaly et al., 2007) since the cheese processor became the largest influent contributor at this time. A broadly similar pattern of increase was observed for average annual effluent TKN concentration (Table 2), although the magnitude of increase was not as large as in the influent. Average effluent TKN was only 12 to 27% of the influent TKN.
Table 2. Annual averages for total Kjeldahl N (TKN including NH 4 +-N), NO 2 – + NO 3 --N, NH 4 +-N, total P, biochemical oxygen demand (BOD), chemical oxygen demand (COD), and total suspended solids (TSS) of the untreated wastewater influent (Infl.) produced by the Las Cruces West Mesa Industrial Park and of the treated wastewater effluent (Effl.) used for plot irrigation in 2002 to 2005. The influent and effluent waters were sampled at the frequencies reported in Table 1 with exceptions noted in footnotes below. The N and P analyses included dissolved plus particulate fractions (analysis on total digest). The standard error of the mean (SE) is reported as an average across all four years.
| Concentration (mg L -1) | ||||||||||||||
| TKN | (NO 2 -+NO 3 - )-N | NH 4 +-N | P | BOD | COD | TSS | ||||||||
| Year | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. | Infl. | Effl. |
| 2002 | 39.2 | 10.7 | 0.3 | 0.2 | 19.0 a | 0.0 a | 19.3 | 7.4 | 227.8 | 32.9 | 397.3 b | 1441.0 b | 335.5 | 108.2 |
| 2003 | 77.4 | 9.6 | 8.7 | 7.6 | 13.0 c | 0.0 c | 38.1 | 12.8 | 353.2 | 30.7 | 971.0 | 225.0 | 777.5 | 157.7 |
| 2004 | 62.0 | 14.4 | 1.5 | 7.9 | 11.4 | 0.2 | 43.9 | 14.9 | 259.6 | 35.9 | 793.2 | 189.6 | 829.6 | 101.0 |
| 2005 | 106.5 | 19.0 | 0.1 | 1.1 | 14.6 | 3.5 | 42.0 | 25.9 | 873.2 | 60.8 | 1766.8 | 352.3 | 1403.5 | 56.7 |
| SE | 16.5 | 2.6 | 1.8 | 1.9 | 6.1 d | 1.3 d | 9.4 | 2.1 | 153.1 | 9.5 | 346.2 | 297.2 | 277.0 | 32.8 |
| aIn 2002, determination made only for month of October. bIn 2002, determinations made only for months of October, November, and December. cIn 2003, determination made only for month of November. dBased only on years 2004 and 2005. |
||||||||||||||
Average annual influent NO 2 – + NO 3 --N concentrations were below 2 mg L -1 for all years except 2003 (Table 2). In the first half of 2003, the influent NO 2 – + NO 3 --N concentration was below detection limit, but in the last half of 2003 the influent NO 2 – + NO 3 --N was 16 and 27 mg L -1 at the last two seasonal measurements, resulting in a high annual SE of 6 mg L -1. Average annual effluent NO 2 – + NO 3 --N concentrations were erratic (Table 2). In 2002 and 2005, effluent NO 2 – + NO 3 --N concentrations averaged less than 6% of effluent TKN. In 2003 and 2004, the average effluent NO 2 – + NO 3 --N concentrations approached 8 mg L -1 and were more comparable to the average effluent TKN concentrations. Average annual effluent NO 2 – + NO 3 --N concentration never exceeded the maximum permissible NO 3 --N concentration of 10 mg L -1 established under the NMED-GWQB permit.
Influent NH 4 +-N (measured in TKN analyses but included in Table 2) ranged between 11 and 19 mg L -1 (Table 2). In 2002, influent NH 4 +-N accounted for about half of the influent TKN concentration. Between 2003 and 2005, influent NH 4 +-N accounted for only 14 to 18% of influent TKN because the influent may have become enriched with organic-N from the cheese processor, overshadowing the NH 4 +-N contribution. The effluent NH 4 +-N concentrations in 2002 and 2003 were below detection limit, and NH 4 +-N was less than 1 mg L -1 when averaged across all years. The relatively high average annual effluent NH 4 +-N in 2005 resulted from a high concentration in January (11 mg L -1), whereas the three remaining quarterly analyses averaged 1 mg L -1. The discord in influent and effluent NH 4 +-N may have resulted from NH 3 volatilization in the lagoons and holding pond.
Average annual influent total P concentration followed a pattern similar to influent TKN concentration in that P increased by at least twofold after 2002 (Table 2). The trend of rising P concentration was not unexpected in view of the high concentrations of P in cheese whey solids (Wendorff and Matzke, 1993). Average effluent P concentration increased by 2 to 11 mg L -1 each year, and in contrast to TKN, effluent P represented a relatively large proportion of the influent P (34 to 62%).
Relatively low influent BOD and COD concentrations (Table 2) compared to other cheese processing plants (Britz et al., 2006) may have resulted from a dilution effect on the cheese processor influent by other WMIP industrial tenants. Average influent BOD and COD concentrations increased 1.6 to 2.4 times from 2002 to 2003, decreased marginally in 2004, and increased considerably in 2005. These changes were generally similar to those for influent TKN noted previously. The BOD concentration averaged 26 to 78 times higher in the cheese processor influent than in the specialty wire manufacturer influent (data not shown). Across the years, the effluent BOD averaged 9% of influent BOD, and for 2003–2005, effluent COD averaged 22% of influent COD. The average for 2002 effluent COD may be unreliable (D. Santantonio, personal communication, 2009) since the October and November COD was 312 to 611 mg L -1, compared to 3400 mg L -1 in December.
There were progressive increases in the average influent TSS concentration (Table 2) that broadly followed the pattern of TKN, BOD, and COD. The average annual TSS concentration in the effluent was 13% of that in the influent, indicating an 87% settling of TSS in the WWTP. Analogous calculations revealed annual reductions of 81% for TKN, 56% for total P, 91% for BOD, and 74% for COD (excluding 2002), indicating effective settling processes in the WWTP.
Effluent irrigation
The average annual nonstressed (100%) ET of L. tridentata and P. glandulosa was 123 to 145 cm, of which effluent supplied 14 to 39% (Table 3). Annual effluent application depths ranged from 21 to 56 cm (820 to 2252 m 3 on the 0.4-ha plot). There were 13 to 17 cm of rainfall in 2002 and 2003 that provided 9 to 13% of nonstressed ET, whereas 2004 and 2005 supplied 24 to 26 cm of rain, or 18 to 19% of nonstressed ET. Thus, annual effluent plus rainfall met an average of 41% of the mean nonstressed shrub ET on the irrigated plot, compared to an average of 15% (rainfall only) on the adjacent non-irrigated plot (Table 3).
Effluent limitations in 2002, 2003, and 2005 were not anticipated and restricted our ability to rigorously test effluent effects on the soil and vegetation. City of Las Cruces personnel revealed the following causes of effluent limitations during the water-limited years: 1) maintenance of a minimum water level in the lagoons and holding pond to protect the pumps and conveyance system (as much as 30% of annual influent supply); 2) effects of summer evaporation from the lagoons and holding pond, which caused disproportionately lower effluent availability; and 3) periodic discharge of cheese process water to another land application site managed by the company tenant.
The mixed vegetation presented additional limitations to irrigation management. There are no published crop coefficients for the herbaceous species at the site, and the seasonal patterns of L. tridentata and P. glandulosa nonstressed ET differ (Ruiz et al., 2006; Babcock et al., 2009). The numerical average of shrub ET, an imperfect measure, underestimates L. tridentata ET and overestimates P. glandulosa ET during winter through spring, while it exceeds L. tridentata ET and underestimates P. glandulosa ET from summer through fall. These limitations were unavoidable due to the irrigation system, mixed vegetation, and lack of available crop coefficients.
Table 3. Annual nonstressed (100%) evapotranspiration (ET) of L. tridentata and P. glandulosa (averaged across both species), annual effluent irrigation to the 0.4-ha irrigated plot, and annual rainfall recorded at the New Mexico Climate Center Fabian Garcia Research Station (2002–2005). Irrigation applies to only the irrigated plot, while rainfall applies to both non-irrigated and irrigated plots.
| Year |
Avg. nonstressed |
Effluent irrigation | Rainfall |
Irrigation + rainfall |
||
| (cm per year) | (% of avg. ET) | (cm per year) | (%of ave. ET) | |||
| 2002 | 132.5 | 32.0 | 24.2 | 16.8 | 12.7 | 36.9 |
| 2003 | 142.8 | 20.5 | 14.4 | 12.9 | 9.0 | 23.4 |
| 2004 | 144.8 | 56.3 | 38.9 | 25.5 | 17.6 | 56.4 |
| 2005 | 123.2 | 33.7 | 27.4 | 23.7 | 19.2 | 46.6 |
Table 4. Annual land deposition of total suspended solids (TSS), N, P, K +, Ca 2+, Mg 2+, Na +, Cl –, and alkalinity by land application of treated effluent to the 0.4-ha irrigated plot (2002–2005). Annual deposition recorded each December as the product of annual effluent land application volume by average annual effluent analyte concentration in Tables 1 and 2 (specific ion data in Table 1 first converted from mol c m -3 to mg L -1). Deposition values are corrected to kg ha -1 basis or 2.5 times the land area of the irrigated plot. Total (bottom line) represents cumulative land deposits from 2002 through 2005.
| Annual and total deposition from effluent irrigation (kg ha -1) | |||||||||||
| Year | TSS | TKN a | NO 2 -+NO 3 --N | Total N b | P | K + | Ca 2+ | Mg 2+ | Na + | Cl - | Alkalinity c |
| 2002 | 346 | 34 | 0.6 | 35 | 24 | 222 | 334 | 78 | 2231 | 3626 | 662 |
| 2003 | 323 | 20 | 15.6 | 35 | 26 | 232 | 52 | 27 | 1334 | 672 | 1353 |
| 2004 | 569 | 81 | 44.5 | 126 | 84 | 550 | 94 | 63 | 3379 | 1377 | 3267 |
| 2005 | 191 | 64 | 3.7 | 68 | 87 | 505 | 40 | 48 | 3044 | 1109 | 3020 |
| Total | 1429 | 199 | 64.4 | 264 | 221 | 1509 | 520 | 216 | 9988 | 6784 | 8302 |
| aTotal Kjeldahl-N (including NH 4 +-N). bSum total of all effluent N fractions measured: total Kjeldahl-N (including NH 4 +-N) and NO 2 – + NO 3 --N. cExpressed as CaCO 3 equivalents. |
|||||||||||
Effluent mineral deposition
The largest annual land deposits of TSS; TKN; NO 2 – + NO 3 --N; total N, K +, and Na +; and total alkalinity were made in 2004 (Table 4) as a result of the high effluent application noted previously. The annual deposition of total N was below the 225 kg ha -1 limit established in the NMED-GWQB permit.
Ion deposition reflected NaCl salinity only in 2002, with the three remaining years reflecting Na + and alkalinity. Through the 4-yr study, 10 Mg of Na +, 6.8 Mg of Cl –, and 8.3 Mg of alkaline anion land deposits were made per ha (Table 4). Fifty-three percent of the 4-yr Cl – deposition and 64% of the 4-yr Ca 2+ deposition occurred in 2002, or prior to influent Cl – reduction and Ca 2+ precipitation noted previously. Over one-third of the cumulative Mg 2+ deposition also occurred in 2002 when the average effluent Mg 2+ concentration was highest.
Ninety-two percent of the alkaline anion deposits occurred between 2003 and 2005. The collective Na +, Cl –, and alkalinity mass accounted for 92% of the total ion deposition (27.3 Mg per ha over 4 yr) when accounting for the smaller 4-yr deposits of K + (1.5 Mg ha -1), Ca 2+ (0.5 Mg ha -1), and Mg 2+ (0.2 Mg ha -1) (Table 4). The average annual ion deposition of around 7 Mg ha -1 is a considerable salt load in agricultural terms (Biggar et al., 1984).
The opposite side of ion deposition is a diversion of salts from conventional WWTPs that direct tertiary water treatment flows into surface waters that, in turn, increase total dissolved solids (TDS) of the surface waters (Anning et al., 2007). The WMIP has an annual wastewater treatment capacity of 548,000 m 3, which is close to the annual 100% shrub ET of around 520,000 m 3 (1.4 m) for the 36-ha site (four times the average annual effluent application of 36 cm and 26% of shrub ET reported in Table 3). At this scale, the annual salt deposition would reach 1000 Mg across the 36-ha site. Given low Rio Grande flow below Las Cruces into El Paso (Texas) of about 220 million m 3 in two of the past ten years (International Boundary and Water Commission, 2003, 2004), this amount of salt deposition would represent a TDS contribution of around 5 mg L -1 to the Rio Grande entering El Paso, which has a median daily TDS of around 700 mg L -1 (Anning et al., 2007). Numerous New Mexico towns are considering development of land application sites, and the nearby reservoirs (Elephant Butte and Caballo) are at 6 to 12% of their capacities at this writing (Natural Resources Conservation Service, 2012b). Thus, the potential for land application to mitigate Rio Grande salinization is apparent, especially in dry years.
Soil analysis
The effluent did not increase soil pH until 2003 under intershrub space at 7.5 cm and under L. tridentata to 45 cm (Figure 1). By 2004 and 2005, soil pH of all irrigated sampling sites increased by as much as a full unit down to 22.5 cm, and under the shrubs there were additional increases down to 45 cm. The irrigated soil pH increases during 2004 and 2005 were associated with increasing deposition of alkalinity (Table 4).
In general, soil organic matter averaged <0.5% throughout the depths of all ground types and for both non-irrigated and irrigated plots, with no major influences from applied effluent (Figure 2). Such relatively low organic matter is characteristic of desert soils (Lajtha and Klein, 1988; MacKay et al., 1987). Following 2004, the year of highest TSS deposition (Table 4), organic matter was 0.1 to 0.2% higher throughout several sampled depths under the irrigated plot intershrub space and P. glandulosa as compared to the non-irrigated plot. In 2005, soil under P. glandulosa at depths from 135–195 cm had 0.7 to 0.8% organic matter compared to about 0.2% in the non-irrigated plot. The higher shallow-depth organic matter under irrigated P. glandulosa is associated with lower bulk density, higher saturated hydraulic conductivity, and higher drainable porosity as compared to irrigated intershrub spaces (Babcock et al., 2009).
Soil TKN ranged from about 50 to 250 mg kg -1 and decreased with depth (Figure 3). In December 2005, TKN averaged 29–34 mg kg -1 higher at most depths below irrigated intershrub space and L. tridentata as compared to the non-irrigated counterparts. However, there was no evidence for a buildup of TKN under any of the irrigated ground types. Also, there was little or no enhancement of soil NH 4 +-N by effluent irrigation up to December 2004, after which soil NH 4 +-N analysis was discontinued (Figure 4).
Total P in soil solids averaged around 100 to 150 mg kg -1 with no consistent effect of effluent irrigation below the shallow depths (Figure 5). In 2004, there were slight elevations in shallow-depth total P under irrigated intershrub spaces and L. tridentata that followed the pattern of Olsen-P discussed next.
In 2002, there was a trend for higher NO 3 --N and Olsen-P concentrations under the non-irrigated sampling sites that received no supplemental N and P (Figures 6 and 7). This may have reflected differential N and P fertility status in the early portion of the study. In 2004, there were marginally higher upper-depth soil NO 3 --N concentrations under irrigated relative to non-irrigated intershrub spaces (about 1 mg kg -1 differences), with a larger increase in soil NO 3 --N (about 4 mg kg -1) under P. glandulosa at 105 cm (Figure 6). Soil NO 3 --N down to 105 cm increased two to 11 times under all irrigated sampling sites in 2005 following a cumulative total N deposition of 264 kg ha -1 (Table 4), with the highest enrichment occurring below the shrubs. However, there was no indication of elevated soil NO 3 --N at the lowest average depth of 195 cm under any of the sampling sites.
Effluent effects on Olsen-P began to appear in 2003 in the uppermost depth under P. glandulosa (Figure 7). When annual P deposition more than tripled during 2004 and 2005 (Table 4), higher Olsen-P was observed at all sampling sites and to as low as 75 cm under L. tridentata. Between 2004 and 2005, upper depth Olsen-P concentration differences between the non-irrigated and irrigated plots had widened by 1 to 5 mg kg -1. As with the soil pH and NO 3 --N increases, there was a tendency for higher effluent-related Olsen-P enrichment below the shrub sites relative to the intershrub space sites in 2005.
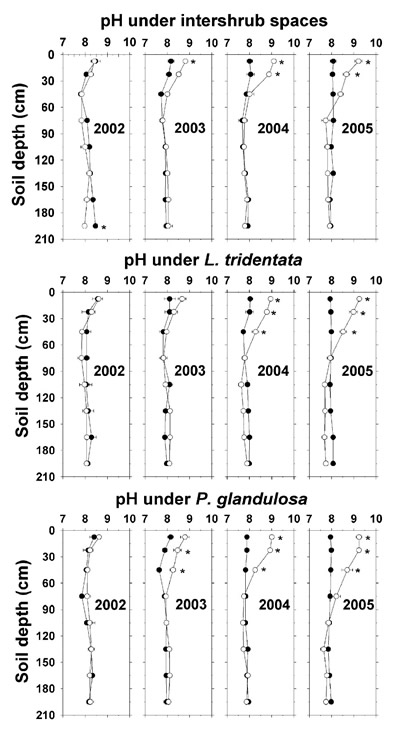
Figure 1. Soil saturation extract pH at average depths from 7.5 to 195 cm at December 2002–2005 under the three ground types of intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Each point is at the average sampled depth of 15- or 30-cm bulk cores and represents the mean + SE of triplicate determinations per sampled site. For some of the means, the SE is smaller than the symbol. Asterisks denote significant differences between the non-irrigated and irrigated plots at P ≤ 0.05 by two-sample t-test within ground type, year, and depth.
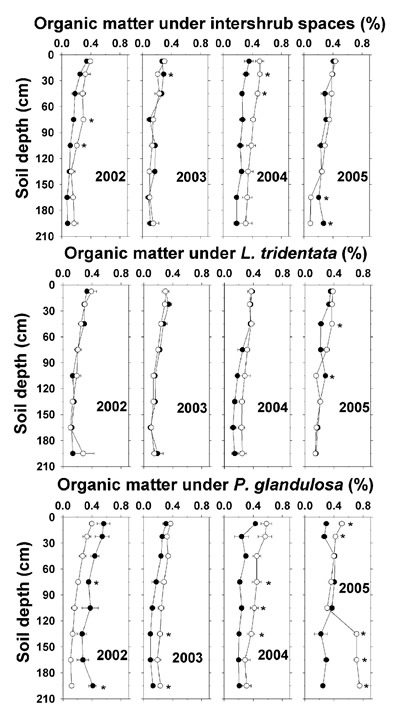
Figure 2. Soil organic matter (dry weight basis) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
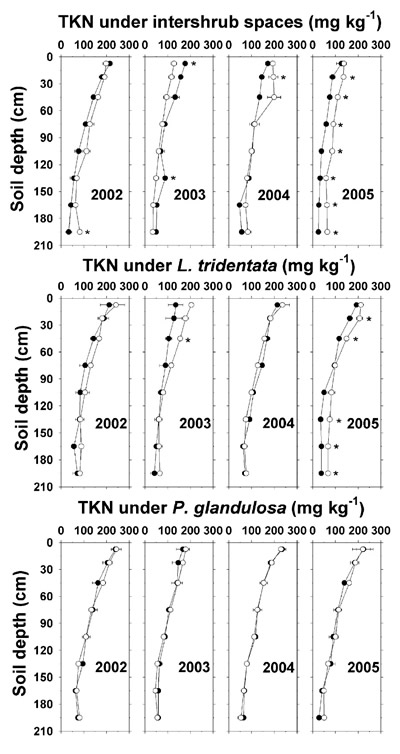
Figure 3. Soil total Kjeldahl-N concentration (TKN, dry weight basis) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
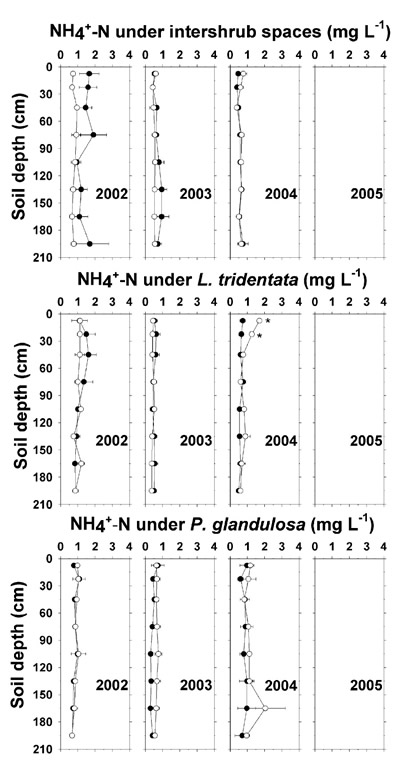
Figure 4. Soil saturation extract NH 4 +-N concentration under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Analyses were discontinued after 2004. Further details as in Figure 1.
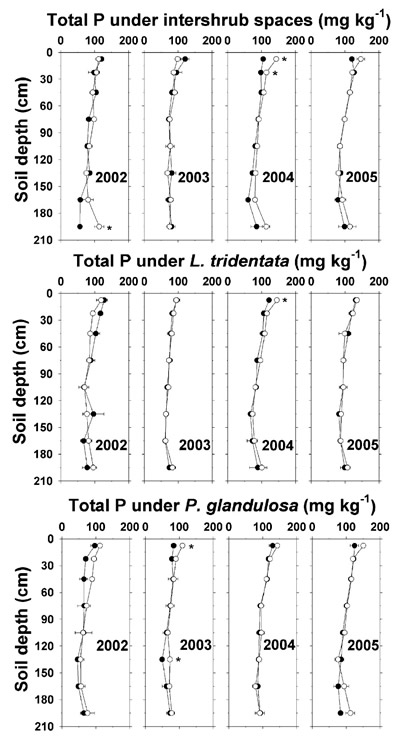
Figure 5. Total P concentration in soil solids (dry weight basis) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
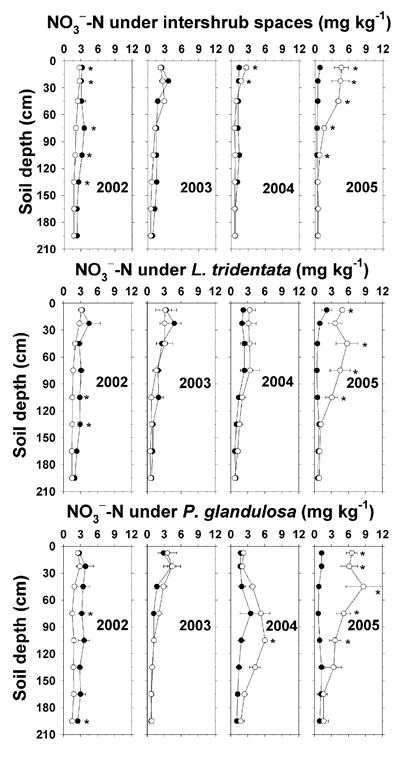
Figure 6. Soil NO 3 --N concentration (dry weight basis) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Saturation extract NO 3 --N (mg L -1) may be estimated by multiplying the plotted averages by 6 to account for the soil saturation percentage. Further details as in Figure 1.
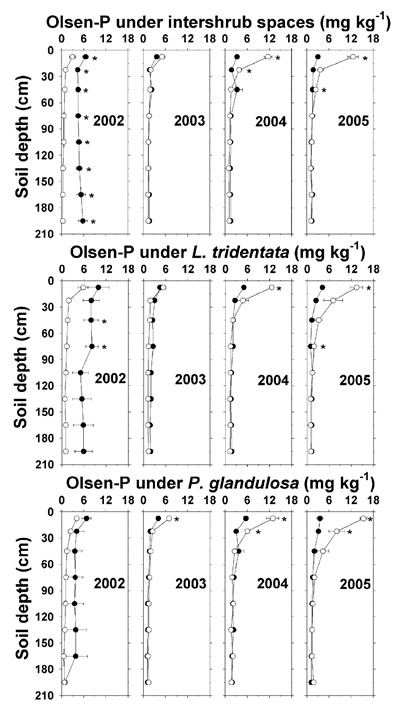
Figure 7. Soil Olsen-P concentration (dry weight basis) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
Between 2003 and 2005, Olsen-P at all irrigated sites increased with depth. Subsurface leaching of P from organic wastes is potentially significant in coarse-textured soils with low P-adsorption capacity (O'Connor et al., 2005). Our data on Bluepoint sand suggest that continuous irrigation with P-containing effluent may induce P leaching. We found no other data on P leaching in semiarid soils receiving land applications of treated wastewater, although in a biosolids study on a semiarid loam soil, Brenton et al. (2007) observed P leaching to the bottom of a 30-cm-tall intact soil column following biosolids P application at 150 kg ha -1 and two rainfall simulation leaching events. In our study, apparent P leaching to 22.5 cm and lower, after a cumulative 4-yr P deposition of 221 kg ha -1 (Table 4), denotes a degree of similarity to the biosolids study. In that study, however, P leaching was detected after about four months, compared to 4 yr in the present field study with smaller P increments from treated effluent.
Effluent irrigation did not affect soil soluble K + concentrations until there was high K + deposition in 2004 and 2005 (Figure 8 and Table 4). By 2004, the effluent increased soluble K + at 7.5 cm depth by 24–33 mg kg -1 and by 14–23 mg kg -1 down to 45 cm under the shrubs, which was consistent with the shrub effects on soil pH, NO 3 --N, and Olsen-P noted previously. In 2005, effluent irrigation effects on soluble K + had diminished, although a higher soluble K + concentration remained in the uppermost depth at the irrigated P. glandulosa site.
On the irrigated plot, the largest increases in saturation extract Na + occurred during the high Na + deposition years of 2004 and 2005 (Figure 9 and Table 4). In 2004, saturation extract Na + under all sampling sites reached a maximum of 23–31 mol c m -3 at 45 cm depth, with significant increases to the bottom of the shrub soil profiles but not under intershrub spaces. In 2005, there were higher saturation extract Na + concentrations throughout most of the soil depths of all irrigated sampling sites, and a trend for higher middle-profile saturation extract Na + under the irrigated shrubs than under irrigated intershrub spaces. In 2004 and 2005, higher saturation extract Na + at 195 cm below the irrigated relative to non-irrigated sites (differences of 4–6 mol c m -3) suggests Na + leaching below the sampled depth range.
Effluent-related increases in saturation extract Ca 2+ (by 5–15 mol c m -3) and Mg 2+ (by 2–5 mol c m -3) were smaller than those for Na +, and were observed at or below 75 cm under P. glandulosa in 2004 and under intershrub spaces and L. tridentata in 2005 (Figures 10 and 11). At the 195 cm depth under irrigated P. glandulosa in 2004 and under irrigated intershrub spaces in 2005, saturation extract Ca 2+ was 5 mol c m -3 higher and saturation extract Mg 2+ was 2–3 mol c m -3 higher than on the non-irrigated plot. As with Na +, Ca 2+ and Mg 2+ may have leached below the sampled profile. A similar finding was reported by Bridgham et al. (1977) in a land application study in the northeastern U.S. involving high-Na + food processing effluent.
By 2003, effluent irrigation increased SAR e under intershrub spaces to 22.5 cm depth (Figure 12). Thereafter, all irrigated sites had elevated SAR e down to at least 45 cm. By the end of the study, surface depth SAR e ranged from 26 to 35 at all sites. Even though the increases in SAR e diminished with depth, they remained apparent down to 165–195 cm under the shrubs by 2005, a pattern not observed under the intershrub spaces.
At the end of the highest Cl - deposition year of 2002 (Table 4), there was little or no increase in the irrigated plot soil saturation extract Cl - concentrations (Figure 13). However, increases in saturation extract Cl - were observed under all irrigated sampling sites and most depths in 2004, after a 3-yr cumulative Cl - deposition of 5.7 Mg ha -1. In that season, Cl - reached as high as 76 mol c m -3 at 105 cm under L. tridentata. A similar pattern was observed in 2005 with 6.8 Mg ha -1 of cumulative Cl - deposition, but with no substantive increases above the 2004 concentrations. At 195 cm depth under the irrigated shrubs in 2004 and under irrigated intershrub spaces in 2005, saturation extract Cl – was 9–14 mol c m -3 higher than in the respective non-irrigated plot samples, indicating that effluent-applied Cl - could have been detected below our sample depth range. High downward mobility of effluent-applied Cl - reveals potential for leaching of NO 3 –-N, another mobile anion, at higher N deposition rates than in our study.
The EC e (Figure 14) followed a pattern similar to that of saturation extract Cl - in that there was a downward advancement in maximum EC e across the years. The highest EC e was observed at the end of the highest combined Na +, Cl -, and alkalinity deposition season of 2004 (Table 4), when at 75–105 cm, EC e reached as high as 4.0–6.1 dS m -1 under irrigated L. tridentata and 4.9–5.4 dS m -1 under irrigated P. glandulosa. The higher ion concentrations at 195 cm below the irrigated plot relative to the non-irrigated plot, as noted previously, were reflected in parallel differences in EC e.
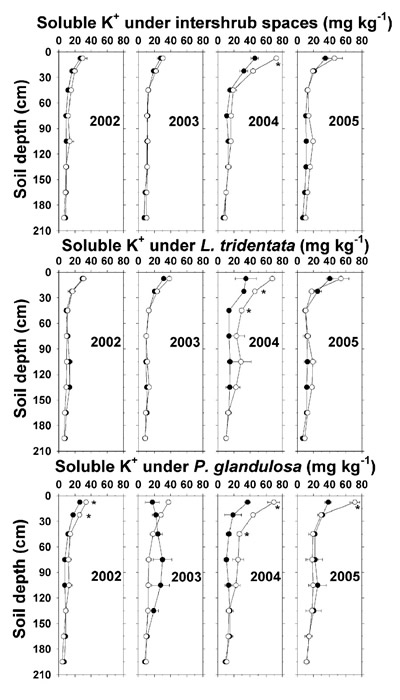
Figure 8. Soil soluble K + concentration (dry weight basis) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Soil saturation extract K + (mg L -1) may be estimated by multiplying the plotted averages by 6 to account for the soil saturation percentage. Further details as in Figure 1.
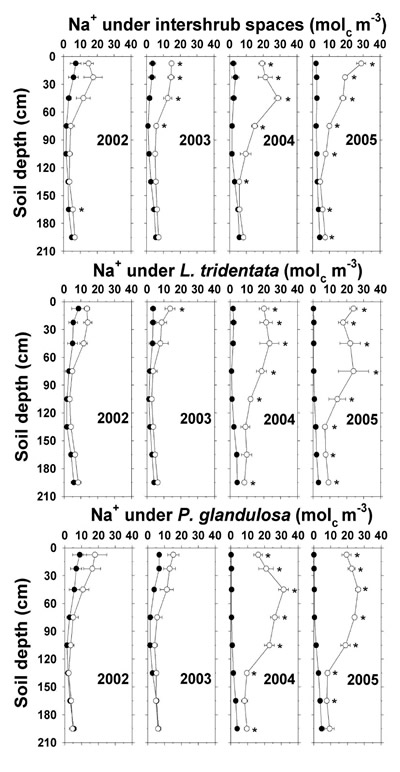
Figure 9. Soil saturation extract Na + concentration under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
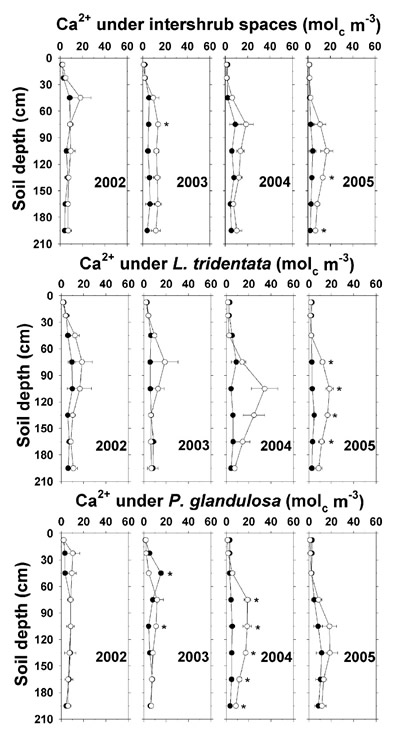
Figure 10. Soil saturation extract Ca 2+ concentration under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
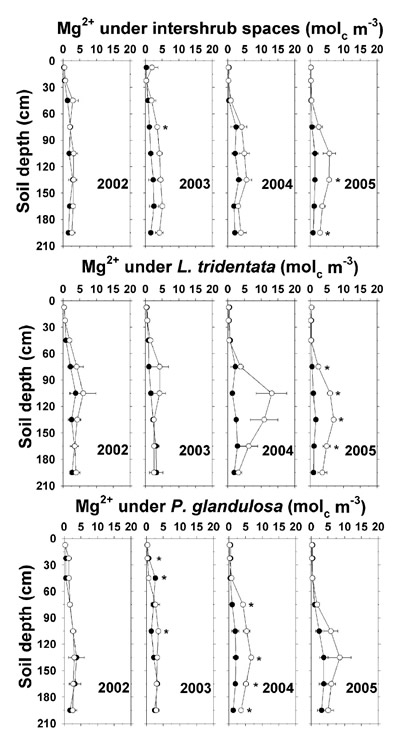
Figure 11. Soil saturation extract Mg 2+ concentration under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
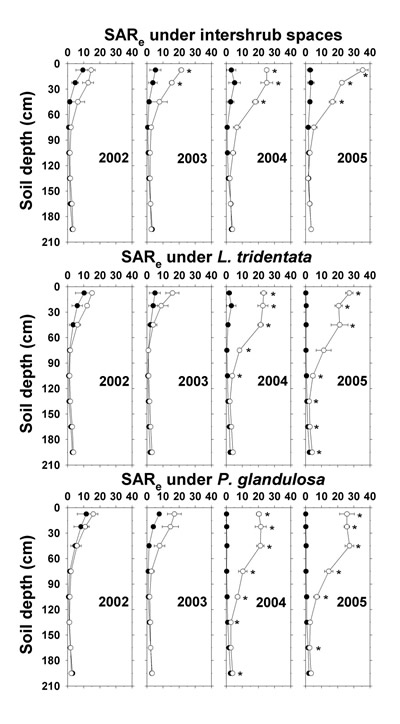
Figure 12. Soil saturation extract sodium adsorption ratio (SAR e) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). SAR calculated as Na +/(Ca 2+ + Mg 2+) 1/2; all ion concentrations in mmol L -1. Further details as in Figure 1.
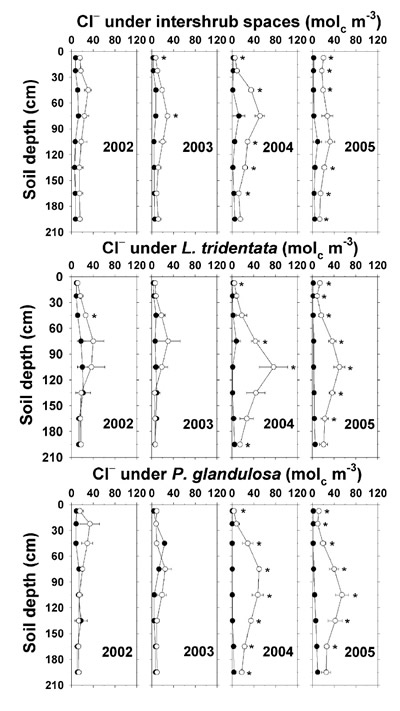
Figure 13. Soil saturation extract Cl – concentration under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
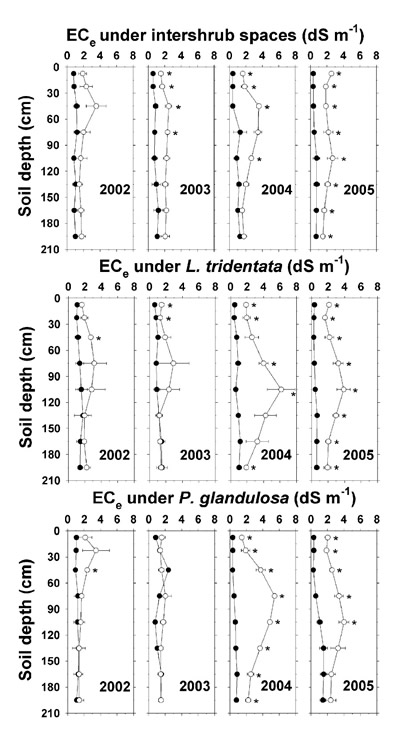
Figure 14. Electrical conductivity of the soil saturation extract (EC e) under intershrub space, L. tridentata, and P. glandulosa, with the non-irrigated plot as the closed symbol (•) and the irrigated plot as the open symbol (°). Further details as in Figure 1.
Depending on irrigated ground type and year, TDC at 60–90 cm depth ranged from 21–44 mol c m -3, while TDA ranged from 28–48 mol c m -3 (data not presented). The fact that, under the irrigated ground types, Cl - alone balanced the entire TDC pool at 60–90 cm from 2003–2005 indicates that the shift in effluent anion composition away from Cl - was not reflected in the saturation extract, even after three years of the high alkaline deposits. Precipitation of effluent-applied CO 3 2– in the irrigated soils was not investigated.
These findings indicate that effluent irrigation increased soil N, P, and K + fertility and the soil stress factors of pH, SAR, Cl –, and EC e, the latter to high levels in agricultural terms (Ayers and Westcot, 1985). During 2004 and 2005 and with increasing depth, soil NO 3 --N, Olsen-P, soluble K +, saturation extract Na + and Cl –, and EC e were observably higher under irrigated L. tridentata and P. glandulosa than under irrigated intershrub spaces. Due to canopy spray interception, the ground area beneath L. tridentata rece ived up to 16% more sprinkler-applied effluent than an equivalent ground area beneath intershrub space (Babcock et al., 2009), which would partially account for these increases. It has been suggested that natural shrub "islands of fertility" in the surface soil (<10 cm depth) have contributed to shrub encroachment and ecosystem change in the Chihuahuan Desert (Cross and Schlesinger, 1999). Our findings suggest that deeper nutrient enrichment under the shrubs would exacerbate shrub competitiveness on Chihuahuan Desert lands receiving wastewater nutrients. Additionally, water extraction by these deep-rooted shrubs (Gibbens and Lenz, 2001) may cause salt to concentrate at low depths (Jarrell and Virginia, 1990b), which is supported by saturation extract data (Na +, Cl –, and EC e) given previously.
Recovery of effluent-applied minerals in soil analysis
Most (≥57%) mineral deposits except K + (8%) and Na + (13%) were recovered in the top 210 cm (Table 5). Soil under intershrub spaces (78% of ground area) accounted for 79 to 93% of the additional amounts of TKN, total P, soluble K +, and saturation extract Ca 2+ and Mg 2+ present on the irrigated plot compared to the non-irrigated plot. The soil below the shrubs (combined 22% of ground area) accounted for disproportionately large contributions of 29 to 38% of the extra NO 3 –-N, Na +, and Cl – on the irrigated plot, as demonstrated in Figures 6, 9, and 13. Excesses in Ca 2+, Mg 2+, Na +, and Cl – began to appear in 2002, whereas the excesses in TKN, NO 3 –-N, total P, and K + first appeared in 2004.
While the cumulative TKN deposition from effluent was only 199 kg ha -1, an excess of over 1000 kg TKN per ha was recovered in the 2.1-m depth soil analysis. This represented a 50% increase in soil TKN content on the irrigated plot, which arose from additive effects of the small numeric differences in December 2005 TKN concentration throughout the depths under intershrub space and L. tridentata, as discussed previously. Excess soil NO 3 –-N of 58 kg ha -1 in the irrigated plot accounted for 90% of cumulative effluent NO 2 –/NO 3 –-N deposits, although that estimate should be viewed with caution. Firstly, we assumed that NO 3 –-N was dominant over NO 2 –-N in the effluent. Secondly, since soil organic matter was less than 1% with no evidence for a progressive effluent-related buildup of soil TKN (Figures 2 and 3), we assumed that there were negligible amounts of N mineralization from organic-N fractions. Whatever the case, there was no evidence for effluent-related NO 3 –-N leaching losses below 2.1 m in our conditions (Figure 6).
A total P excess of 173 kg ha -1 in the irrigated soils accounted for 78% of the cumulative P deposition. The soluble K + excess of 122 kg ha -1 represented only 8% of the cumulative K + deposition since the analysis excluded unavailable soil K + fractions. The Ca 2+ excess of 654 kg ha -1 was 26% higher than cumulative Ca 2+ deposition. The Mg 2+ excess of 151 kg ha -1 accounted for 70% of the cumulative Mg 2+ deposition, and the Cl – excess of nearly 4 Mg ha -1 corresponded to 57% of the cumulative Cl – deposition. Unaccounted for Mg 2+ and Cl – likely leached below the 2.1-m profile. The Na + excess of around 1.3 Mg ha -1 was only 13% of cumulative Na + deposition, with the remainder apparently below the profile and in the unmeasured exchangeable fraction.
Table 5. December 2005 soil mineral content (kg ha -1 of TKN, NO 3 --N, total P in solids, and soluble K +, Ca 2+, Mg 2+, Na +, and Cl –) in the adjacent 0.4-ha non-irrigated and irrigated plots, and absolute difference in 2005 soil mineral content (mean irrigated minus mean non-irrigated plots) expressed in kg ha -1 and as a percentage of cumulative 4-yr (2002–2005) mineral deposition by effluent irrigation from bottom line entries of Table 4. Mineral content calculated by summation of the products of mineral concentration and dry soil weight through all sampled depths of the 2.1-m soil profile, accounting for proportion of land area occupied by intershrub spaces, L. tridentata, and P. glandulosa shrubs, and correcting to one ha total land area.
| Soil mineral content | ||||||||
| Irrigation treatment | TKN | NO 2 --N | P | K + | Ca 2+ | Mg 2+ | Na + | Cl - |
| Non-irrigated (kg ha -1) a | 2074 ± 170 | 19 ± 2 | 3345 ± 69 | 473 ± 51 | 368 ± 82 | 71 ± 15 | 340 ± 70 | 812 ± 312 |
|
Irrigated (kg ha -1) a |
3121 ± 140 | 77 ± 11 | 3518 ± 177 | 595 ± 74 | 1022 ± 174 | 222 ± 28 | 1595 ± 61 | 4674 ± 622 |
| Difference (kg ha -1) | 1047 | 58 | 173 | 122 | 654 | 151 | 1255 | 3862 |
| Difference (% of effluent) | 526 | 90 b | 78 | 8 | 126 | 70 | 13 | 57 |
| aEach value is the mean ± SE of three observations. bEffluent analysis included NO 2 –-N with NO 3 –-N. |
||||||||
Intershrub space herbaceous species biomass and mineral accumulation
On average, one to three herbaceous species were identified on the intershrub space sampling frames in October, depending on year and irrigation treatment (Figure 15). Herbaceous species populations on both the non-irrigated and irrigated plots were dynamic in total species number (species richness), total shoot dry matter (TDM), and species composition. These changes were coincident with soil-related modifications on the irrigated plot and with increases in annual rainfall (Table 3). On the non-irrigated plot, TDM reached 64 to 73 g m -2 with the relatively high rainfall in 2004 and 2005, but was only 14 g m -2 with low annual rainfall in 2002 (Figure 15). After 2002, herbaceous species diversity also increased on the non-irrigated plot, with average species numbers of 1.2 ± 0.1 in 2002, 2.7 ± 0.3 in 2004, and 2.3 ± 0.3 in 2005. Unlike the non-irrigated plot, the irrigated plot experienced progressive increases in herbaceous TDM and a general decline in species richness between 2002 and 2005. The irrigated plot TDM averaged 57, 97, and 145 g m -2, and the number of species declined from 2.9 ± 0.2 to 2.1 ± 0.3 to 1.2 ± 0.1 in 2002, 2004, and 2005, respectively.
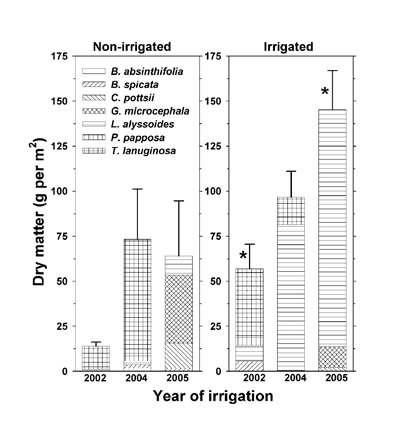
Figure 15. Aboveground dry matter of herbaceous vegetation from randomly positioned 1-m 2 frames of the non-irrigated and irrigated plots harvested on October 4, 2002; October 21, 2004; and October 26, 2005. The grams per m 2 values multiplied by 7.8 provide kg dry matter per ha corrected for percentage of land area in intershrub spaces that supported the herbaceous vegetation (78% of land area in both non-irrigated and irrigated plots). Bar components (identified in legend) are average dry matter per individual species. Total bar height represents the average ± SE for total frame dry matter (all species combined). An asterisk above an irrigated plot average denotes significant difference in total frame dry matter from the non-irrigated plot average within same year by two-sample t-test at P ≤ 0.05.
Tidestromia lanuginosa was the dominant herbaceous species on the non-irrigated plot in 2002 and 2004 (88% to 91% of average TDM). It was not detected on the non-irrigated plot in 2005, when G. microcephala became dominant (59% of TDM), followed by C. pottsii, L. alyssoides , and B. spicata (22%, 17%, and 2% of TDM, respectively). Gutierrezia microcephala has low water use efficiency compared to other southwestern U.S. desert perennial species (Sandquist et al., 1993), supporting the possibility that the increased rainfall in 2004 and 2005 allowed this species to dominate the non-irrigated stand in 2005. Of the 2002 irrigated plot TDM, T. lanuginosa made up 67%, with the remaining third from about equal contributions by B. spicata, L. alyssoides , and P. papposa. Thereafter, L. alyssoides became dominant on the irrigated plot; T. lanuginosa contributed only 16% to irrigated TDM in 2004 and was not present on that plot in 2005.
The decline in T. lanuginosa may have been related to increased competition caused by increased rainfall that, by 2005, favored G. microcephala, C. pottsii, and L. alyssoides on the non-irrigated plot, and that, by 2004 and 2005, favored L. alyssoides (and to a lesser degree G. microcephala) on the irrigated plot. Supplemental water and higher soil N, P, and K +, along with higher soil salinity, sodicity, and pH, may also have caused the species changes on the irrigated plot. Under irrigation, L. alyssoides became increasingly dominant, contributing 16% to TDM in 2002, but 84 to 90% in 2004 and 2005.
Our study does not elucidate the cause of L. alyssoides growth enhancement under irrigated conditions in 2004–2005. However, findings on other Lepidium spp. in the western U.S. are noteworthy. Lepidium latifolium L. and L. densiflorum Schrad. are known to aggressively occupy sites that are affected by high salinity or alkalinity (Francis and Warwick, 2007; Halvorson and Lang, 1989). Even though published data on L. alyssoides are limited, our results involving this species are consistent with the reports on other Lepidium spp. Proliferation of L. alyssoides on the irrigated plot during 2004 and 2005 occurred when the intershrub space shallow depth soil saturation extract pH increased to 9 and higher (Figure 1) and shallow depth SAR e increased to 25–35 (Figure 12), their highest levels of the 4-yr study. In September 2004, soil saturation extract Na + reached 40 mol c m -3 at the 0–30 cm depth (data not shown), which could have equated to around 80 mol c m 3 even at the upper end of the field moisture range (U.S. Salinity Laboratory Staff, 1954).
There was a twofold difference in cumulative TDM (2002, 2004, and 2005) between the non-irrigated and irrigated plots for all herbaceous species combined, even though the annual difference in 2004 did not reach statistical significance due to a variable average on the non-irrigated plot (Figure 15). Cumulative 3-yr TDM was 151 g m -2 on the non-irrigated plot and 299 g m -2 on the irrigated plot, corresponding to a shoot TDM excess of 1154 kg ha -1 on the irrigated plot following the 3-yr period. During warm months, the irrigated intershrub spaces were virtually covered with herbaceous vegetation, especially L. alyssoides in 2004 and 2005. On the non-irrigated plot, herbaceous vegetation was sparse and patchy throughout the time period.
Generally, the contents (kg ha -1) of TKN, P, K +, Ca 2+, Mg 2+, Na +, and Cl – in the aboveground herbaceous tissues of the irrigated intershrub spaces were higher than the amounts in the counterpart non-irrigated herbaceous shoot tissues (Table 6). For seven of the 21 year x mineral two-sample irrigation treatment comparisons, the t-test did not reach statistical significance. The TKN concentrations of the herbaceous vegetation ranged from 1.14 to 2.32%, with no observable differences between non-irrigated and irrigated plots. The 2004 and 2005 TKN concentrations in the irrigated plot (mainly L. alyssoides ) were 2.16% and 1.26%, respectively, which may be attributed to the relatively low effluent total N deposition in 2005 compared to 2004 (68 and 126 kg ha -1, respectively; Table 4), thus limiting the shoot N yield by the irrigated vegetation in 2005 despite the high shoot dry matter yield. The cumulative difference in total N content of the herbaceous vegetation of 18 kg ha -1 (Table 6) accounted for about 8% of the 2002, 2004, and 2005 effluent total N deposition of 229 kg ha -1 (Table 4). On a mineral weight basis, comparatively small cumulative surpluses in the irrigated plot were observed for herbaceous tissue P, K +, Ca 2+, and Mg 2+ contents (2 to 9 kg ha -1; Table 6), representing less than 2% of the accrued 3-yr deposition of P, K +, Ca 2+, and Mg 2+ (Table 4). The largest cumulative surpluses in the herbaceous vegetation (weight basis) were for Na + and Cl – (20 and 26 kg ha -1, respectively; Table 6), although those surpluses represented less than 1% of the cumulative 3-yr Na + and Cl – deposition (Table 4). Cumulative mineral surpluses as percentages of 4-yr mineral deposition (not shown in Table 6) differed little if at all from the 3-yr deposition basis (6.8% for total N, 1.3% for P, 0.4% for K +, 1.6% for Ca 2+, 1.1% for Mg 2+, and no differences for Na + and Cl –).
Table 6. Aboveground (shoot) TKN, P, K +, Ca 2+, Mg 2+, Na +, and Cl – content of herbaceous plants (kg ha -1) from non-irrigated and irrigated plots on October 4, 2002; October 21, 2004; and October 26, 2005. Mineral contents per ha are corrected for percentage of total land area in intershrub space that supported the herbaceous vegetation (78% of land area in both non-irrigated and irrigated plots). Cumulative difference across years (irrigated minus non-irrigated plots) expressed as kg ha -1 and as percentage of effluent mineral deposition during the seasons of vegetation analysis excluding 2003 deposition (from Table 4). For convenience, cumulative 3-yr effluent irrigation deposition values (kg ha -1) inserted here as follows: 229 (total N), 195 (P), 1277 (K +), 468 (Ca 2+), 189 (Mg 2+), 8654 (Na +), and 6112 (Cl –).
| Mineral content (kg ha -1) | Cumulative Difference | |||||
| Mineral | Irrigation treatment a | 2002 | 2004 | 2005 | kg ha -1 | % of mineral deposition |
| TKN | Non-irrigated | 2.4 | 5.2 | 11.2 | --- | --- |
| Irrigated | 5.9* b | 15.7* | 15.2 ns c | 18.0 | 7.9 | |
| P | Non-irrigated | 0.2 | 0.4 | 0.8 | --- | --- |
| Irrigated | 0.5* | 1.6* | 2.2* | 2.9 | 1.5 | |
| K + | Non-irrigated | 4.7 | 5.6 | 8.6 | --- | --- |
| Irrigated | 11.4* | 9.0 ns | 5.1 ns | 6.7 | 0.5 | |
| Ca 2+ | Non-irrigated | 1.7 | 6.8 | 6.7 | --- | --- |
| Irrigated | 6.4* | 7.7 ns | 9.6 ns | 8.5 | 1.8 | |
| Mg 2+ | Non-irrigated | 0.7 | 2.2 | 0.9 | --- | --- |
| Irrigated | 2.4* | 1.9 ns | 2.0* | 2.4 | 1.3 | |
| Na + | Non-irrigated | 0.1 | 0.5 | 0.0 | --- | --- |
| Irrigated | 2.1 ns | 9.1* | 9.1* | 19.6 | 0.2 | |
| Cl - | Non-irrigated | 3.0 | 2.0 | 2.0 | --- | --- |
| Irrigated | 15.4* | 10.4* | 10.4* | 26.2 | 0.4 | |
| aValues for non-irrigated and irrigated plot entries are in kg ha -1, as mean ± SE of 10 randomly positioned 1-m 2 frames, and calculated as product of mineral concentration of overall frame (as a percentage of the dry matter of all species pooled together) by total frame dry matter (all species together, from Figure 15). bAsterisk denotes significant difference within year and mineral by two-sample t-test at P ≤ 0.05. cNon-significant (ns) by two-sample t-test at P ≤ 0.05. |
||||||
Across the three sampling dates, Cl – concentration of the herbaceous species tissues averaged 1.88% in the irrigated plot compared to 1.17% in the non-irrigated plot. The irrigated vegetation Cl – was highest in 2002 (3.85%) and decreased to 1.00% in 2004 and to 0.80% in 2005. The progressive decline in shoot Cl – concentration, and to some degree Cl – excess on the irrigated plot, occurred with decreasing effluent Cl – deposition (3636 to 1109 kg ha -1 from 2002 to 2005, respectively; Table 4), and as T. lanuginosa gave way to L. alyssoides . The cumulative surplus for Na + was most obvious in 2004 and 2005 when L. alyssoides dominated the species composition. Sodium concentrations in the non-irrigated plot tissues averaged 0.13% in 2002, 0.06% in 2004, and 0.01% in 2005. By contrast, the average Na + concentration of the irrigated vegetation was 0.36% in 2002, and when L. alyssoides later dominated in 2004–2005, Na + increased to 0.72 to 1.14%. The increasing Na + represents a major difference to the decreasing Cl - concentrations reported previously.
The non-irrigated herbaceous vegetation K +:Na + molar ratio was relatively high and variable with the mixed vegetation, remaining at or above 52:1 throughout the study (Figure 16). By contrast, the K +:Na + ratio on the irrigated plot was only 5.8:1 in 2002, and as the irrigated herbaceous species composition shifted from T. lanuginosa to L. alyssoides in 2004–2005, the ratio decreased to between 0.5:1 and 0.8:1, by which time there was no shoot K + surplus on the irrigated plot (Table 6). Decrease in the irrigated plot vegetation K +:Na + ratio was due to both the increased shoot Na + concentrations noted previously and to major reductions in shoot K + concentration (2.90%, 1.34%, and 0.47% of dry weight in 2002, 2004, and 2005, respectively). Conspicuous declines in already low shoot K +:Na + ratios under the irrigated, high-Na + conditions are characteristic of Na +-tolerant, natrophilic plants, within which increasing Na + concentrations are associated with decreasing K + concentrations, and wherein Na + may substitute for metabolic roles of K + (Greenway and Munns, 1980; Flowers and Läuchlii, 1983). These findings suggest that ground cover enhancement and effluent mineral uptake by L. alyssoides could be of particular value for desert land application systems that release high-Na + effluent, although species richness may decline with the encroachment.
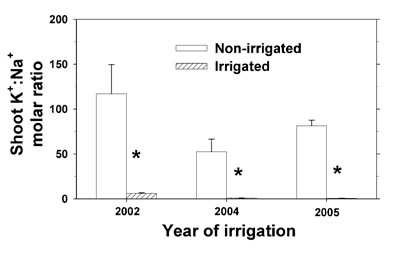
Figure 16. Mixed herbaceous vegetation K +:Na + molar ratio from harvest dates listed in Figure 15. On the irrigated plot, T. lanuginosa represented 67% of TDM in 2002, while L. alyssoides represented 84–90% of TDM in 2004–2005 (see Figure 15). In some cases, the SE is too small to be visible. Asterisks above irrigated plot averages denote significant differences from the non-irrigated plot within year by two-sample t-test at P ≤ 0.05.
Shrub whole canopy biomass and mineral accumulation
There were no changes in shrub numbers, mean shrub canopy diameter, and percentage of shrub cover for either L. tridentata or P. glandulosa throughout the study period. For shrub number and diameter, the non-irrigated and irrigated plot averages, respectively, were as follows: 66 and 71 L. tridentata shrubs with mean canopy diameters of 2.5 ± 0.1 m and 2.3 ± 0.1 m, and 35 and 27 P. glandulosa shrubs with mean canopy diameters of 4.1 ± 0.3 m and 4.4 ± 0.5 m. The main branch tissues (all wood wider than 0.5 cm in diameter) averaged 79% of the total L. tridentata canopy biomass and 95% of the total P. glandulosa canopy biomass. We obtained the following averages for shrub TDM per unit PCA after multiplying by the fraction of plot area covered by the canopies: 119 ± 12 g m -2 for non-irrigated L. tridentata , 167 ± 1 g m -2 for irrigated L. tridentata, 138 ± 19 g m -2 for non-irrigated P. glandulosa, and 136 ± 23 g m -2 for irrigated P. glandulosa. These averages are within the fall to winter dried biomass per ground area ranges reported in previous Chihuahuan Desert studies (Huenneke et al., 2001, 2002; Miller and Huenneke, 2000a).
There were no differences (P ≤ 0.05) in PCA versus biomass (TDM) regression slope or elevation between the non-irrigated L. tridentata shrubs harvested in 2002, 2004, and 2005; between the non-irrigated 2004 and 2005 P. glandulosa harvests; or between the irrigated 2004 and 2005 P. glandulosa harvests. Thus, the three years of non-irrigated L. tridentata canopy biomass data (n = 15 shrubs) and the two years of non-irrigated and irrigated P. glandulosa canopy biomass data (n = 10 shrubs) were combined into three overall regressions for further analysis (Figure 17 and Table 7). In southern New Mexico conditions, Huenneke et al. (2001) relied upon 4-yr composite regressions for L. tridentata and P. glandulosa biomass estimations (in 1-m 2 quadrats), while Molinar et al. (2002) stated that linear regressions pooled across three years best described P. glandulosa land cover and density. Minimal year-to-year variation in total shrub biomass reflected the inherently slow growth responses of these shrub species under Chihuahuan Desert conditions (Buonopane et al., 2005; Huenneke et al., 2001; Allen et al., 2008; Miller and Huenneke, 2000b; Khumalo et al., 2008).
The PCA explained 85 to 98% of the variability (coefficient of determination; R 2) in canopy TDM for non-irrigated and irrigated L. tridentata and for irrigated P. glandulosa (Figure 17). For non-irrigated P. glandulosa, TDM for the two largest shrubs sampled in 2005 was variable, resulting in an R 2 of only 0.52 for the combined years of 2004 and 2005 (2002 data excluded from the 2005 regression analysis although they increased the R 2 by 0.10). The R 2 for non-irrigated P. glandulosa in 2004 (n = 5 shrubs) was 0.94 compared to a value of 0.38 for 2005 (n = 5 shrubs). Thus, all but two of the 51 harvested shrubs effectively contributed to R 2 values of 0.85 or higher.
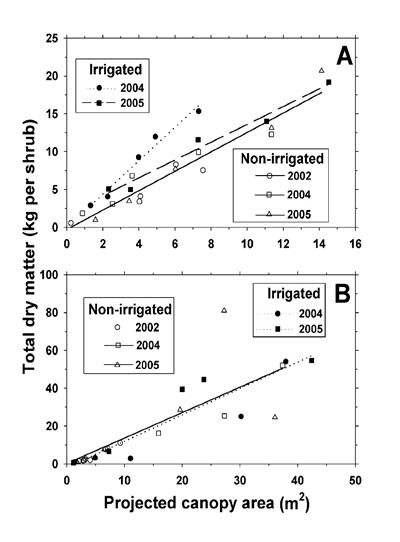
Figure 17. Relationship between total aboveground dry matter per shrub (TDM) and projected canopy area (PCA) of L. tridentata (A) and P. glandulosa (B) measured in dormant seasons of February 2002, March 2004, and March 2005. Each point represents a single shrub. L. tridentata regression equations: non-irrigated (2002, 2004, and 2005 combined; solid line and open symbols): TDM = –0.2721 + 1.2822PCA, R 2 = 0.93, n = 15; irrigated (2004; dotted line and closed symbol): TDM = –0.0399 + 2.2087PCA, R 2 = 0.97, n = 5; irrigated (2005; dashed line and closed symbol): TDM = 1.8369 + 1.1761PCA, R 2 = 0.98, n = 5. P. glandulosa regressions (2004 and 2005 combined): non-irrigated (solid line and open symbols): TDM = 0.2323 + 1.3444PCA, R 2 = 0.52, n = 10; irrigated (dotted line and closed symbols): TDM = –1.9417 + 1.3942PCA, R 2 = 0.85, n = 10. The 2002 non-irrigated P. glandulosa line (points only, excluded from non-irrigated regression) is as follows: TDM = –2.4046 + 1.4461PCA, R 2 = 0.96, n = 6.
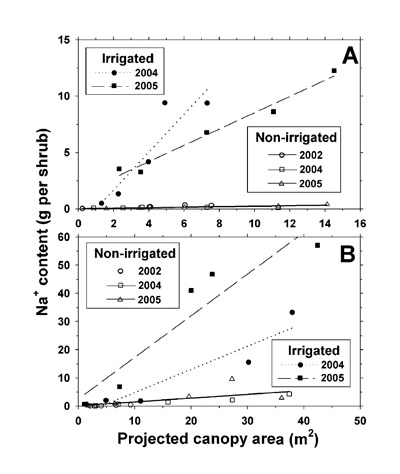
Figure 18. Relationship between total aboveground Na + content per shrub and projected canopy area (PCA) of L. tridentata (A) and P. glandulosa (B) measured in dormant seasons of February 2002, March 2004, and March 2005. Each point represents a single shrub. L. tridentata regression equations: non-irrigated (2002, 2004, and 2005 combined; solid line and open symbols): Na + = 0.0357 + 0.0200PCA, R 2 = 0.51, n = 15; irrigated (2004; dotted line and closed symbol): Na + = –1.7102 + 1.6827PCA, R 2 = 0.85, n = 5; irrigated (2005; dashed line and closed symbol): Na + = 1.2905 + 0.7228PCA, R 2 = 0.98, n = 5. P. glandulosa regressions: non-irrigated (2004 and 2005 combined; solid line and open symbols): Na + = 0.0994 + 0.1354PCA, R 2 = 0.41, n = 10; irrigated (2004; dotted line and closed symbol): Na + = –3.3799 + 0.8196PCA, R 2 = 0.89, n = 5; irrigated (2005, dashed line and closed symbol): Na + = 2.4568 + 1.4784PCA, R 2 = 0.89, n = 5. The 2002 non-irrigated P. glandulosa line (points only, excluded from non-irrigated regression) is as follows: Na + = –0.0791+ 0.0482PCA, R 2 = 0.96, n = 6.
By March 2004 (after two years of effluent irrigation), the slope of TDM rise per unit increase in PCA was almost twice as high for irrigated L. tridentata as for non-irrigated L. tridentata (Figure 17 and Table 7). While this trend was not apparent in 2005, the F-test for elevation difference (numerically higher in the irrigated plot than in the non-irrigated plot) nearly reached statistical significance. Lack of significant 2005 canopy biomass stimulation of irrigated L. tridentata was unexpected. March 2005 biomass was determined just following the 2004 growing season, during which the irrigated plot received as much effluent and rainfall (82 cm) and 68% and 80% more P and total N (84 and 126 kg ha -1, respectively) as did the 2002 and 2003 growing seasons combined (Tables 3 and 4). Yet the slope of the 2004 irrigated L. tridentata line (just following the 2003 growing season) was greater than that of the 2005 irrigated L. tridentata line (Figure 17 and Table 7). The 2-yr regression lines for non-irrigated and irrigated P. glandulosa TDM were nearly identical (Figure 17 and Table 7).
Whole shrub data are rarely reported in the Chihuahuan Desert literature and are the only means of estimating whole plant biomass. There was little indication that effluent application stimulated total shrub canopy biomass. More importantly, there was no suppression in total canopy biomass after three years of land applying the saline–sodic irrigation water. This is a critical factor for long-term viability of the shrubs. Using regression analysis with the plot shrub survey, the following dried biomass estimates were obtained at March 2005: 1531 and 1875 kg ha -1 for irrigated L. tridentata and P. glandulosa, respectively, and 1087 and 1754 kg ha -1 for non-irrigated L. tridentata and P. glandulosa, respectively. Thus, by March 2005, a total shrub canopy tissue biomass excess of 565 kg ha -1 was detected under irrigated relative to non-irrigated conditions, with 79% of the excess from L. tridentata.
Two-sample t-tests (P ≤ 0.05) within shrub species and tissue and across the years of 2002–2005 revealed no changes in the concentrations of TKN, P, K +, Ca 2+, Mg 2+, Na +, and Cl – in shrub canopy tissues of the non-irrigated plot. There were also no differences between the non-irrigated and irrigated plots in TKN, P, K +, Ca 2+, and Mg 2+ concentrations in shrub canopy tissues of either species at either March 2004 or March 2005. High Na + and Cl – deposition was reflected in significant differences in shrub canopy tissue Na + and Cl – concentrations between the non-irrigated and irrigated plots. Thus, further discussion on mineral concentrations is limited to Na + and Cl –.
Under non-irrigated conditions, the shrub Na + concentrations were no higher than 0.05% in any year. In irrigated L. tridentata, the 2004 and 2005 Na + concentrations averaged between 0.02 and 0.05% in main branches, 0.17 and 0.18% in leaves, and 0.08 and 0.12% in twigs. In irrigated P. glandulosa, the 2004 and 2005 Na + concentrations averaged 0.06 to 0.10% in main branches and 0.12 to 0.15% in twigs. The only significant effects of effluent irrigation on shrub Cl – concentrations were observed in March 2004. At that time, twig and leaf Cl – concentrations of irrigated L. tridentata were about double those of non-irrigated L. tridentata (0.62% twig Cl – and 0.88% leaf Cl – on the irrigated plot compared with 0.33% twig Cl – and 0.46% leaf Cl – on the non-irrigated plot). In P. glandulosa twigs, 2004 Cl – concentrations averaged 0.36% and 0.26% on the irrigated and non-irrigated plots, respectively. The Cl – concentrations in shrub main branches were relatively low and unaffected by effluent irrigation.
There were no changes in Na + and Cl – concentrations in the non-irrigated shrub tissues throughout the 4-yr study; thus, we combined the 2002, 2004, and 2005 shrub size (PCA) versus Na + and Cl – weight per shrub data for non-irrigated L. tridentata (n = 15 shrubs), and the 2004 and 2005 PCA versus Na + and Cl – weight per shrub data for non-irrigated P. glandulosa (n = 10 shrubs) (Figures 18 and 19, Table 7). Also, there were no significant differences in regression slope or elevation for irrigated P. glandulosa PCA versus Cl – weight per shrub between the 2004 and 2005 seasons; thus, those data were also pooled (Figure 19 and Table 7). As with P. glandulosa TDM data in 2002, we excluded the 2002 data from the regression analysis for Na + and Cl – weight per shrub even though the data increased the R 2 values by 0.10 to 0.12.
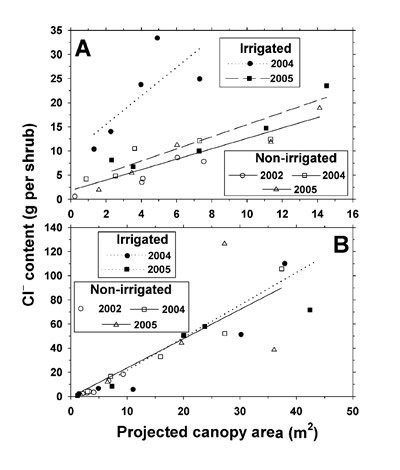
Figure 19. Relationship between total aboveground Cl – content per shrub and projected canopy area (PCA) of L. tridentata (A) and P. glandulosa (B) measured in dormant seasons of February 2002, March 2004, and March 2005. Each point represents a single shrub. L. tridentata regression equations: non-irrigated (2002, 2004, and 2005 combined; solid line and open symbols): Cl – = 1.8493 + 1.0777PCA, R 2 = 0.80, n = 15; irrigated (2004; dotted line and closed symbol): Cl – = 9.6137 + 2.9536PCA, R 2 = 0.57, n = 5; irrigated (2005; dashed line and closed symbol): Cl – = 2.9077 + 1.2548PCA, R 2 = 0.89, n = 5. P. glandulosa regressions (2004 and 2005 combined): non-irrigated (solid line and open symbols): Cl – = –0.4041 + 2.4132PCA, R 2 = 0.59, n = 10; irrigated (dotted line and closed symbols): Cl – = –4.6348 + 2.2856PCA, R 2 = 0.86, n = 10. The 2002 non-irrigated P. glandulosa line (points only, excluded from non-irrigated regression) is as follows: Cl – = –3.9824 + 2.3959PCA, R 2 = 0.96, n = 6.
With increasing PCA, there was little increase in Na + weight per shrub under non-irrigated conditions, but there were major increases in Na + weight per shrub under irrigated conditions (Figures 18A and 18B). Highly significant differences in regression slopes (PCA versus Na + weight per shrub) between non-irrigated and irrigated plots were observed at both 2004 and 2005 and for both L. tridentata and P. glandulosa (Table 7). The average slopes for irrigated P. glandulosa and L. tridentata were 9 and 60 times higher, respectively, than under the non-irrigated conditions. The 2004 slope for irrigated L. tridentata was greater than that of the 2005 irrigated slope, which resulted from the biomass differential mentioned previously. The 2005 elevation for irrigated P. glandulosa was higher than the 2004 elevation due to a difference in main branch Na + concentration (0.06% and 0.10% of dry weight in 2004 and 2005, respectively).
Table 7. Probability (P) values from F-tests for linear regression slope and elevation comparisons between non-irrigated and irrigated L. tridentata and P. glandulosa whole canopy aerial tissues harvested in dormant seasons of February 2002, March 2004, and March 2005. The independent variable was shrub projected canopy area (PCA in m 2) and the dependent (response) variables were total aerial dry matter (TDM in kilograms), Na + (in grams), and Cl – (in grams) per shrub. Between-year regressions within species and irrigation treatment that did not differ in slope or elevation were pooled as follows: non-irrigated L. tridentata, all response variables (2002, 2004, and 2005; n = 15 shrubs); non-irrigated P. glandulosa, all response variables (2004 and 2005; n = 10 shrubs); and irrigated P. glandulosa, TDM and Cl – content per shrub (2004 and 2005; n = 10 shrubs). All other regression lines consisted of 5 shrub observations. The 2002 P. glandulosa data were omitted from the non-irrigated regression analyses. Regression lines, coefficients of determination, and equations are shown in Figures 17–19.
| Response variable | |||||||
| TDM (kg per shrub) | Na + (g per shrub) | Cl -(g per shrub) | |||||
| Shrub species | Regressions compared | Slope | Elevation | Slope | Elevation | Slope | Elevation |
| L. tridentata | Non-irrigated a vs irrigated (2004) | 0.0102 | 0.0003 | 0.0000 | 0.0001 | 0.0363 | 0.0000 |
| Non-irrigated a vs irrigated (2005) | 0.5384 | 0.0863 | 0.0000 | 0.0000 | 0.5353 | 0.0783 | |
| Irrigated (2004) vs irrigated (2005) | 0.0057 | 0.0744 | 0.0288 | 0.3590 | 0.2182 | 0.0084 | |
| P. glandulosa | Non-irrigated vs irrigated (2004, 2005) b | 0.9196 | 0.8439 | --- | --- | 0.8680 | 0.5174 |
| Non-irrigated c vs irrigated (2004) | --- | --- | 0.0004 | 0.0233 | |||
| Non-irrigated c vs irrigated (2004) | --- | --- | 0.0001 | 0.0007 | |||
| Irrigated (2004) vs irrigated (2005) | --- | --- | 0.1065 | 0.0192 | |||
| aPooled regression data of non-irrigated plot for all response variables in 2002, 2004, and 2005 (n = 15 each). bPooled regression data of non-irrigated or of irrigated plots for TDM and Cl – content per shrub in 2004 and 2005 (n = 10 each). cPooled regression data of non-irrigated plot for Na + content per shrub in 2004 and 2005 (n = 10 each). |
|||||||
As PCA increased, there were substantial increases in Cl – weight per shrub in both non-irrigated and irrigated plots (Figures 19A and 19B). The average slope for irrigated L. tridentata was only about twice that of non-irrigated L. tridentata (Figure 19A). Thus, the Cl – findings contrast the previously discussed results for Na +. This distinction suggests differential Na + and Cl – uptake characteristics, since under non-irrigated conditions, the soil saturation extract Na + and Cl – concentrations within a 2-m depth were markedly similar throughout the study (generally <10 mol c m -3; Figures 9 and 13). The greater slope of 2004 PCA versus Cl – weight per shrub for irrigated L. tridentata compared with non-irrigated L. tridentata (Table 7) resulted from both higher biomass and Cl – concentrations (twigs and leaves) on the irrigated plot, as noted previously. Otherwise, the patterns of L. tridentata PCA versus Cl – weight per shrub and PCA versus TDM per shrub were similar. A lower regression elevation for irrigated L. tridentata at 2005 compared with 2004 (Figure 19A and Table 7) was associated with a decline in Cl – concentrations of all canopy tissues from 2004 to 2005 (average reduction of 0.11% across all three tissue types). A similar trend in Cl – concentrations was observed for irrigated P. glandulosa (average reduction of 0.14% in two tissue types from 2004 to 2005), although the irrigation treatment regressions did not differ (Figure 19B and Table 7).
Since there were no changes in non-irrigated shrub canopy mineral concentrations across the years, we used the pooled non-irrigated regressions (2002, 2004, and 2005 for L. tridentata; 2004 and 2005 for P. glandulosa) to estimate the shrub canopy mineral contents in the non-irrigated plot. On both plots, there were generally high R 2 values for PCA versus mineral weights per shrub considering the limited shrub sample size (R 2 = 0.76 to 0.98). However, for non-irrigated P. glandulosa, low R 2 values (0.41 to 0.59) reflected the high biomass variability noted previously. Shrub mineral excesses on the irrigated plot were detected for all minerals analyzed and taken as a consequence of cumulative mineral deposition between 2002 and 2004 (Table 4), i.e., effluent mineral recovery. The following excesses (combined for L. tridentata and P. glandulosa) were observed: TKN, 7.3 kg ha -1; P, 0.6 kg ha -1; K +, 1.6 kg ha -1; Ca 2+, 14.2 kg ha -1; Mg 2+, 0.3 kg ha -1; Na +, 3.1 kg ha -1; and Cl –, 0.4 kg ha -1. The excess TKN and Ca 2+ contents represented 3% and 4% of the cumulative 3-yr deposition of TKN and Ca 2+, respectively. The excess P, K +, Mg 2+, Na +, and Cl – contents represented <1% of the cumulative deposition of P, K +, Mg 2+, Na +, and Cl –. The Na + excess was only 0.04% despite the major differences in shrub Na + content between the non-irrigated and irrigated plots previously discussed.
Shrub terminal branch biomass and mineral accumulation
The annual aboveground organs (leaves and fruit) are concentrated on the shrub terminal branches. We anticipated that, in response to effluent application, transient changes in shrub biomass would be more apparent on the terminal branches than in the perennial-dominant structures of the shrub canopy. Biomass stimulation was, in fact, more apparent in the shrub terminal branch tissues, although the enhancement was largely confined to fruit and was time-dependent upon the species. Only the biomass of terminal branch fruit is presented, although wood and leaf dry weights are briefly discussed next.
Effluent irrigation did not affect total leaf area per branch (166 to 327 cm -2 and 197 to 232 cm -2 for L. tridentata and P. glandulosa, respectively) or specific leaf weight (24 to 25 mg cm -2 for both species). Terminal branch wood dry weight (averaging 10.9 g for L. tridentata and 6.8 g for P. glandulosa) was also unaffected by effluent irrigation. With one exception, there was no consistent irrigation effect on terminal branch leaf dry weights (3.8 to 7.9 g and 1.2 to 5.9 g for L. tridentata and P. glandulosa, respectively). At the October 2002 and October 2004 sample dates, terminal branch leaf dry weight of P. glandulosa averaged 44% less on the irrigated plot than on the non-irrigated plot (4.7 versus 3.2 g per branch in 2002, 3.5 versus 1.4 g per branch in 2004 on non-irrigated and irrigated plots, respectively; P ≤ 0.05). The basis of the latter finding is unclear due to lack of relevant published data on these shrubs. For example, while effluent irrigation increased P. glandulosa fruit production considerably (see following paragraph) and high crop loads may accelerate leaf senescence in cultivated trees (Smith, 1976; Sparks, 1977; Picchioni et al., 1997), we are unaware of any analogous findings on L. tridentata or P. glandulosa. High Na + accumulation in P. glandulosa leaves on the irrigated plot (see later discussion) may have resulted in metabolic stress with accelerated leaf senescence, but critical leaf Na concentrations for P. glandulosa are not available in the literature.
Early summer terminal branch fruit dry weights of non-irrigated and irrigated L. tridentata averaged 2.7 to 3.4 g per branch up to the 2004 sample date, with no indication of an effluent irrigation effect until the 2006 sample date (Figure 20A). On that date, average fruit dry weight of non-irrigated L. tridentata declined by 1.5 g per branch from the average at the previous two early summer sample dates. By contrast, the L. tridentata fruit dry weight on the irrigated plot reached 4.4 g per branch in early summer 2006, about three times the average on the non-irrigated plot. Fruit dry weights of non-irrigated P. glandulosa (Figure 20B) increased progressively, which may have resulted from the increased rainfall in 2004 and 2005. There were substantial increases in irrigated P. glandulosa fruit dry weight across the early summer sampling dates, suggesting additive effects of effluent irrigation. In early summer 2004 and 2006, fruit dry weights on irrigated P. glandulosa branches were 4 to 14 times higher than on the non-irrigated plot. That difference was also apparent in July 2002 (P = 0.0637). Despite the lack of available effluent after January 2006, the June 2006 fruit growth stimulation on the shrubs may have been aided by deep residual soil moisture from fall 2005 (Babcock et al., 2009) and by the shallow to moderate depth increases in soil nutrients (Figures 6–8). This conclusion is supported by the findings of Lajtha and Whitford (1989) and Lajtha (1987) for L. tridentata; relevant field data for P. glandulosa are lacking. In addition, our results are consistent with those of Fisher et al. (1988), who reported a doubling of terminal branch fruit numbers on L. tridentata receiving supplemental water and N in Chihuahuan Desert conditions.
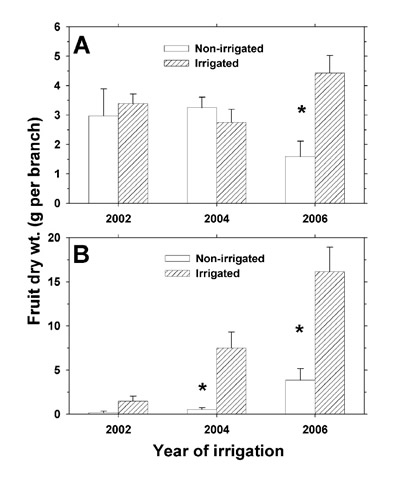
Figure 20. Fruit dry weight on terminal (30-cm-long) branches of L. tridentata (A) and P. glandulosa (B), harvested from the non-irrigated and irrigated plots on July 1, 2002; July 14, 2004; and June 22, 2006. Each bar represents the average + SE of five shrubs, with each shrub consisting of 4 terminal branch observations or 20 total observations. An asterisk for a given year denotes significant difference in fruit dry weight between non-irrigated and irrigated plots by two-sample t-test at P ≤ 0.05.
The highest shrub total branch dry weights were observed on the irrigated plot in June 2006 (18.4 ± 1.4 g and 22.7 ± 1.2 g for non-irrigated and irrigated L. tridentata, respectively, and 13.4 ± 1.7 g and 26.8 ± 2.6 g for non-irrigated and irrigated P. glandulosa, respectively; P ≤ 0.05). The 4.3-g and 13.4-g differences were attributed to the higher fruit dry weights on the irrigated plot. The following values for terminal branch TDM per ha were obtained in June 2006: 425 and 247 kg ha -1 for irrigated L. tridentata and P. glandulosa, respectively, and 281 and 141 kg ha -1 for non-irrigated L. tridentata and P. glandulosa, respectively. Thus, in 2006, a species-combined biomass excess of 250 kg ha -1 was present on the irrigated plot relative to the non-irrigated plot.
Results of the July 2004 leaf washing test for irrigated shrub terminal branch samples (n = 5 shrubs averaged across 7 minerals analyzed) showed that mineral recovery in the washed L. tridentata leaf extracts was 99.7 ± 2.8% of the unwashed L. tridentata leaf extracts. Mineral recovery for irrigated P. glandulosa averaged 87.7 ± 2.1%, indicating that, on average, about 12% of the mineral excess on irrigated P. glandulosa leaves reported in the following paragraphs could have been attributed to superficial (easily washed) mineral residues adhering to the leaf surfaces and not to intercellular or intracellular spaces within the leaf tissue. That is, the foregoing data on P. glandulosa may slightly overestimate the physiologically relevant mineral fractions.
Effluent irrigation had little effect on the concentrations of TKN, P, K +, Ca 2+, and Mg 2+ in shrub terminal branch tissues. Higher soil NO 3 --N, Olsen-P, and soluble K + concentrations that were largely limited to the upper 1-m depth of the irrigated profile (Figures 6–8) may have been partially inaccessible to these deep-rooted shrubs (Gibbens and Lenz, 2001). On the other hand, previous Chihuahuan Desert studies reported a lack of foliar N and P increase in response to N and P fertilization for L. tridentata in field conditions (Lajtha and Whitford, 1989; Lajtha, 1987), and for P. glandulosa in a controlled environment (Maestre and Reynolds, 2006). Larrea tridentata is thought to "thrive" in high-Ca 2+ soils (Cross and Schlesinger, 1999), and the June 2006 L. tridentata leaf Ca 2+ concentration was significantly higher on the irrigated plot (2.25%) than on the non-irrigated plot (1.57%) (data not presented), presumably in response to the elevated soil Ca 2+ in 2005 (Figure 10). We do not know the extent to which the elevated 2006 leaf Ca 2+ contributed to the increased 2006 L. tridentata fruit production (Figure 20A), although the higher leaf Ca 2+ contributed to effluent Ca 2+ recovery on the irrigated plot, as reported later in our discussion.
The basis of fruiting enhancement by effluent irrigation noted previously, particularly the earlier fruiting response by P. glandulosa compared with L. tridentata, cannot be clearly determined from our data, including the variation in leaf nutrient concentrations. Elevated soil NO 3 –-N concentrations were detected 1 yr earlier under irrigated P. glandulosa than under irrigated L. tridentata (Figure 6), but there was no consistent pattern to suggest that increased fruiting resulted from increased concentrations of leaf N or, realistically, from any other measured nutrient in either L. tridentata or P. glandulosa. Irrigation application rate was based on the average of L. tridentata and P. glandulosa ET, and leading up to the early summer terminal branch sampling dates, irrigation supplied a greater proportion of P. glandulosa full ET than of L. tridentata full ET (Ruiz et al., 2006; Babcock et al., 2009). Water availability is a major determinant of stomatal behavior, CO 2 assimilation rate, and productivity of L. tridentata and P. glandulosa (Nilsen et al., 1981; Hamerlynck et al., 2004). In L. tridentata, CO 2 assimilation per leaf area is strongly correlated with stomatal conductance but poorly correlated with leaf N content per leaf area (Barker et al., 2006), lending support to the water-limitation hypothesis. Further study is needed to elucidate the relative importance of water and nutrients to the increases in irrigated shrub fecundity observed in this study.
Effluent irrigation increased terminal branch leaf Na + and Cl - concentrations at most sampling dates (Tables 8 and 9; only early summer data shown). Leaf Na + and Cl - concentrations tended to be higher than those in wood and fruit. The highest leaf Na + concentrations were observed during the high Na + deposition year (2004), when leaves on irrigated L. tridentata had 0.64% in July and leaves on irrigated P. glandulosa had 0.77% in October. Irrigated L. tridentata leaves appeared to have higher leaf Cl - concentrations than irrigated P. glandulosa leaves, although both species had the highest leaf Cl - concentration during high Cl - deposition in 2002, reaching 2.09% and 1.61%, respectively, in October. However, the progressive increases in soil saturation extract Cl - concentrations on the irrigated plot (Figure 13) did not result in further increases in shrub leaf Cl - concentrations.
Interestingly, early summer leaf Cl - concentrations of the non-irrigated shrubs were high (0.53 to 1.11%) considering the low soil saturation extract Cl - concentrations in the non-irrigated plot (Figure 13). In July 2004, we estimated that these leaf Cl - concentrations on a tissue water basis were 150–160 mol m -3, whereas Na + in the tissue water was <10 mol m -3 (data not presented). These Cl - concentrations are on the order of those previously reported in the tissue water of numerous halophytes growing under saline conditions in semiarid Utah (Wiebe and Walter, 1972). That Cl - contributes to osmotic adjustment in these shrubs is an intriguing possibility worthy of further study, although there may be an upper limit to leaf Cl - tolerance.
In cultivated tree crops, leaf Na + and Cl - concentrations as high as those reported previously (0.77% Na +, 2.09% Cl -) are associated with foliar toxicity symptoms, growth reductions, or loss in root function (Bernstein, 1980; Miyamoto et al., 1985; Picchioni et al., 1990, 1991; Picchioni and Graham, 2001). For irrigated P. glandulosa, the low leaf dry weights in October 2002 and October 2004 noted previously were associated with relatively high leaf Na + concentrations (0.46–0.77%). Otherwise, there was no clear indication of harmful leaf Na + and Cl - accumulation in the shrubs, and no salt toxicity symptoms were observed. Critical leaf Na + and Cl - concentrations for L. tridentata and P. glandulosa are not established; thus, it is difficult to determine whether leaf Na + and Cl - accumulations were injurious to the shrubs. Possibly, the phreatophytic trait of these shrubs served as an avoidance mechanism for high Na + proportions in the relatively shallow depths of the irrigated plot (Figure 12).
On the irrigated plot, the early summer shrub terminal branch fruit and wood Na + concentrations tended to be higher than on the non-irrigated plot (Table 8). A similar pattern was observed for L. tridentata fruit and wood Cl - concentrations, although in the P. glandulosa fruit and wood tissues, early summer Cl - concentrations did not differ between the non-irrigated and irrigated plots (Table 9).
Table 8. Sodium concentrations in terminal (30-cm-long) branch tissues of L. tridentata and P. glandulosa under non-irrigated and irrigated conditions, harvested in early summer of 2002 (July 1), 2004 (July 14), and 2006 (June 22). a
| Na + concentration (% of dry weight) |
|||||
| Species | Year | Irrigation treatment | Leaves | Fruit | Wood |
| L. tridentata | 2002 | Non-irrigated | 0.06 | 0.03 | 0.03 |
| Irrigated | 0.04 | 0.03 | --- | ||
| Signifigance b | ns | --- | --- | ||
| 2004 | Non-irrigated | 0.01 | 0.01 | 0.00 | |
| Irrigated | 0.64 | 0.32 | 0.14 | ||
| Signifigance | * | * | * | ||
| 2006 | Non-irrigated | 0.01 | 0.01 | 0.01 | |
| Irrigated | 0.14 | 0.01 | 0.05 | ||
| Signifigance | * | --- | * | ||
| P. glandulosa | 2002 | Non-irrigated | 0.02 | 0.02 | 0.02 |
| Irrigated | 0.04 | 0.02 | 0.04 | ||
| Signifigance | ns | --- | * | ||
| 2004 | Non-irrigated | 0.01 | 0.01 | 0.01 | |
| Irrigated | 0.51 | 0.12 | 0.19 | ||
| Signifigance | * | * | * | ||
| 2006 | Non-irrigated | 0.00 | 0.01 | 0.01 | |
| Irrigated | 0.03 | 0.02 | 0.13 | ||
| Signifigance | * | * | * | ||
| aValues are the means of five shrubs, each comprised of four individual branch observations (20 total branches per mean). Wood data for irrigated plot in 2002 were not available. bNon-significant (ns) or significant (*) at P ≤ 0.05 by two-sample t-test. |
|||||
Table 9. Chloride concentrations in terminal (30-cm-long) branch tissues of L. tridentata and P. glandulosa under non-irrigated and irrigated conditions, harvested in early summer of 2002 (July 1), 2004 (July 14), and 2006 (June 22). a
| Cl - concentration (% of dry weight) |
|||||
| Species | Year | Irrigation treatment | Leaves | Fruit | Wood |
| L. tridentata | 2002 | Non-irrigated | 1.11 | 0.66 | 0.36 |
| Irrigated | 1.72 | 0.71 | --- | ||
| Signifigance b | ns | ns | --- | ||
| 2004 | Non-irrigated | 0.55 | 0.51 | 0.16 | |
| Irrigated | 1.29 | 0.77 | 0.29 | ||
| Signifigance | * | * | * | ||
| 2006 | Non-irrigated | 0.53 | 0.43 | 0.24 | |
| Irrigated | 0.98 | 0.64 | 0.34 | ||
| Signifigance | * | * | * | ||
| P. glandulosa | 2002 | Non-irrigated | 1.01 | 0.70 | 0.60 |
| Irrigated | 1.46 | 0.90 | 0.53 | ||
| Signifigance | * | ns | ns | ||
| 2004 | Non-irrigated | 0.81 | 0.67 | 0.28 | |
| Irrigated | 0.98 | 0.66 | 0.26 | ||
| Signifigance | * | ns | ns | ||
| 2006 | Non-irrigated | 0.74 | 0.57 | 0.25 | |
| Irrigated | 0.94 | 0.43 | 0.25 | ||
| Signifigance | ns | ns | --- | ||
| aValues are the means of five shrubs, each comprised of four individual branch observations (20 total branches per mean). Wood data for irrigated plot in 2002 were not available. bNon-significant (ns) or significant (*) at P ≤ 0.05 by two-sample t-test. |
|||||
June 2006 average mineral contents per branch on the irrigated shrubs generally exceeded those on the non-irrigated shrubs (Table 10). For both shrub species combined, there were relatively large TKN, K +, Ca 2+, and Cl - excesses on the irrigated plot. The estimated terminal branch mineral excesses per ha in both species combined were as follows: 4.3 kg total N, 0.3 kg P, 2.4 kg K +, 2.7 kg Ca 2+, 0.3 kg Mg 2+, 0.4 kg Na +, and 2.0 kg Cl -. About 6 to 7% of 2005 effluent total N and Ca 2+ deposition was accounted for in the irrigated shrub terminal branches. However, less than 1% of the 2005 effluent-deposited P, K +, Mg 2+, Na +, and Cl - was accounted for by the P, K +, Mg 2+, Na +, and Cl - terminal branch excesses. Except for Na +, Cl -, and Ca 2+ tissue concentration effects on excess mineral content noted previously, excesses in the other minerals resulted from the increased fruit dry matter production.
Table 10. Weights of TKN, P, K +, Ca 2+, Mg 2+, Na +, and Cl – in terminal (30-cm) branches (leaves + fruit + wood) of L. tridentata and P. glandulosa under non-irrigated and irrigated conditions, harvested June 22, 2006. Excess mineral content (irrigated plot minus non-irrigated plot) at bottom of table represents the combined contributions of both shrub species to elevated mineral content resulting from effluent irrigation. a
| Mineral content (mg per branch) | ||||||||
| Shrub species | Irrigation treatment | TKN | P | K + | Ca 2+ | Mg 2+ | Na + | Cl - |
| L. tridentata | Non-irrigated | 256.6 | 19.6 | 192.5 | 261.7 | 21.8 | 1.8 | 63.3 |
| Irrigated | 356.6* b | 26.5 ns c | 250.7 ns | 339.7* | 33.0* | 15.8* | 137.4* | |
| P. glandulosa | Non-irrigated | 233.7 | 18.2 | 123.6 | 160.3 | 23.2 | 1.0 | 68.8 |
| Irrigated | 501.0* | 38.6* | 270.4* | 293.1* | 36.8* | 12.2* | 132.8 ns | |
| Excess mineral content | 367.3 | 27.3 | 205.0 | 210.8 | 24.8 | 25.2 | 138.1 | |
| aEach value is the mean of 5 shrubs, with each shrub consisting of 4 individual branch observations (20 total branches per mean). bAsterisk denotes significant difference within species and mineral by two-sample t-test at P ≤ 0.05. cNon-significant (ns) by two-sample t-test at P < 0.05. |
||||||||
Vegetation summary: Biomass, mineral recovery, and final appearance
We estimated biomass and mineral recovery over three years of effluent irrigation for the intershrub space herbaceous species and shrub canopies, and for one year of effluent irrigation for the shrub terminal branches (Table 11). The 3-yr cumulative herbaceous species biomass excess was about twice that of the 3-yr cumulative shrub canopy biomass excess. The 1-yr estimate for shrub terminal branch biomass excess in 2006 (mainly in fruit) was less than half that of the 3-yr shrub canopy biomass excess. The terminal branch estimates must be viewed with caution since they involved only one growing season, and we could not determine fruit biomass and mineral accumulation from shrub carbohydrate and mineral reserves or from soil mineral availability from previous growing seasons. Nonetheless, approximately 2 Mg ha -1 of additional biomass were produced by the three types of vegetation across the 4-yr period.
Table 11. Vegetation comparison for estimated excesses in total dry matter (TDM) and minerals, defined as total dry matter or mineral weight in irrigated plot minus non-irrigated plot. Mineral excesses are expressed as percentages of cumulative effluent irrigation mineral deposition in 2002, 2004, and 2005 for herbaceous species; cumulative deposition of 2002, 2003, and 2004 for shrub canopy tissue; and 2005 annual deposition for shrub terminal branches. Effluent mineral deposition available in Table 4. Herbaceous species mineral estimates are copied from Table 6 to allow comparison and are of mixed vegetation at three sampling dates, and shrub estimates (also in text) are at one sampling date each, with combined totals from both L. tridentata and P. glandulosa.
| Mineral excess (% of mineral deposition through effluent irrigation) |
||||||||||
| Vegetation type | Mineral deposition years |
Sampling date |
TDM excess |
N a | P | K + | Ca 2+ | Mg 2+ | Na + | Cl - |
| Herbaceous | 2002, 2004, 2005 | Oct. 2002, 2004, 2005 | 1154 | 7.9 | 1.5 | 0.5 | 1.8 | 1.3 | 0.20 | 0.40 |
| Shrub canopies | 2002, 2003, 2004 | March 2005 | 565 | 3.7 | 0.5 | 0.2 | 3.0 | 0.2 | 0.04 | 0.01 |
| Shrub terminals | 2005 | June 2006 b | 250 | 6.4 | 0.4 | 0.5 | 6.7 | 0.7 | 0.01 | 0.18 |
| aVegetation N was measured as TKN; deposition N defined as effluent TKN (including NH 4 +-N) plus (NO 2 – + NO 3 –)-N. bMineral excesses expressed as percentages of 2005 annual mineral deposition from 34 cm effluent irrigation. The irrigated plot received 4.6 cm effluent with unaccounted for mineral deposits between January 1, 2006, and the June 22, 2006 sampling date; those deposits were not included in the mineral excess percentage calculations. |
||||||||||
The shrub contributions to N excesses accounted for around 10% of total N deposition despite low shrub density. Much higher densities of these shrubs have been reported in the Chihuahuan, Mojave, and Sonoran Deserts (Reynolds et al., 1999; Cross and Schlesinger, 1999; Jarrell and Virginia, 1990b; Craig and Abella, 2008; Lajtha, 1987; Ettershank et al., 1978; Fisher et al., 1988; Miller and Huenneke, 2000a, 2000b). Approximately 18% of total N deposition was detected in the combined vegetation analyses. The shrub Ca 2+ excesses accounted for 10% of Ca 2+ deposition, with an additional 2% contribution by the herbaceous species Ca 2+ excess. The shrubs could have utilized more Ca 2+ had the effluent not become Ca 2+-limited since Ca 2+ is thought to be limiting to shrubs in saline–alkali desert ecosystems (James et al., 2005). The irrigated vegetation was relatively ineffective in recovering effluent P, K +, Mg 2+, Na +, and Cl -, as those combined mineral excesses accounted for no more than around 2% of the deposition.
Biomass and mineral recoveries in Table 11 are minimal estimates. Firstly, the low-density shrub populations at the WMIP match the description of Chihuahuan Desert shrub populations that function below carrying capacity and that are on the verge of becoming invasive and highly competitive against non-shrub species (Miller and Huenneke, 2000a; Molinar et al., 2002). The question thus arises as to how much higher the shrub N and Ca 2+ recovery could reach with higher shrub densities. In north-central Texas, P. glandulosa can provide up to 100% land cover while producing about 17 times more total dry matter and storing about 25 times more total N per ha than P. glandulosa on our Chihuahuan Desert WMIP irrigated plot (Teague et al., 2008; Hughes et al., 2006). Secondly, the Table 11 averages exclude root contributions. Based on the L. tridentata and P. glandulosa literature, mostly from controlled environments (Perkins and Owens, 2003; Allen et al., 2008; Nilsen et al., 1986; Jarrell and Virginia, 1990a; Bachelet et al., 1986; Lajtha and Klein, 1988), roots of the irrigated shrubs likely represented significant reservoirs of dry matter and land-applied minerals that were not assessed in the study.
The final appearance of the vegetation (June 22, 2006) is shown in Figure 21. The relatively dense intershrub space herbaceous cover on the irrigated plot (mainly L. alyssoides ) (compare Figure 21A with Figure 21D) was largely senescent due to limited effluent availability between January and June 2006. By contrast, the fruit production of irrigated L. tridentata (compare Figure 21B with Figure 21E) and of irrigated P. glandulosa (compare Figure 21C with Figure 21F) illustrates high phenotypic plasticity in response to near-dryland conditions of 2006, which is characteristic of these shrub species (Huenneke et al., 2002; Khumalo et al., 2008; Sharifi et al., 1990; Fisher et al., 1988).
Figure 21. Herbaceous and shrub vegetation at close of study on June 22, 2006. Representative images of the non-irrigated plot (A, B, C) and irrigated plot (D, E, F) include intershrub spaces (top row), L. tridentata terminal branches (middle row), and P. glandulosa terminal branches (bottom row).
Implications for vegetation community changes
Intensified industrial activity is expected to accelerate changes in shrubland communities in the southwestern U.S. (McArthur and Kitchen, 2007), and this 4-yr study of a novel anthropogenic disturbance—land application of treated industrial wastewater—serves as an example. In the Chihuahuan Desert, long-term desertification through shrub encroachment into grasslands has altered biogeochemical cycles of soil nutrients (Cross and Schlesinger, 1999). This has led to concerns over rangeland viability (Allen et al., 2008; Schlesinger et al., 1996; Reynolds et al., 1999; Huenneke et al., 2001, 2002; Molinar et al., 2002; Miller and Huenneke, 2000a; Gibbens et al., 2005), and land managers must weigh that concern against human needs for cost-effective wastewater treatment such as land application. In the present study, land-applied effluent accentuated two shrub-related desertification processes in southwestern deserts, namely the moderate soil depth enrichment of NO 3 –-N, Olsen-P, and soluble K + at the shrub sampling sites (e.g., the "resource island" effect) and increased shrub fecundity. Both L. tridentata and P. glandulosa have relatively low ANPP in comparison to grassland-dominated Chihuahuan Desert communities (Huenneke et al., 2002; Cox et al., 2006). Thus, the 3- to 14-fold increases in shrub fruit dry matter, associated with increases in availability of soil resources, could ultimately have a significant bearing on shrub ANPP. The shrubs create focal points of biological activity under their canopies that regulate Chihuahuan Desert ecosystem structure and function (Cross and Schlesinger, 1999; Maestre and Reynolds, 2006; Aguiar and Sala, 1999). This natural trait could be exploited in the attenuation of soil-applied treated wastewater components. In addition, the shrubs intercept the sprinkler-applied effluent by their canopies (Babcock et al. 2009), which may further intensify the resource island effect.
Still more dramatic than the increased shrub fruit production was the rapid intershrub space vegetation response that was atypical of natural Chihuahuan Desert community dynamics. In the natural state, intershrub spaces have become increasingly barren and deprived due to preferential distribution of resources to the shrub fertility islands (Cross and Schlesinger, 1999; Reynolds et al., 1999), leading to a considerably long-lasting dominance of shrubs over the other vegetation (Cox et al., 2006). Even with less than one year of effluent irrigation and before L. alyssoides dominated the irrigated intershrub spaces, aboveground herbaceous species biomass was four times higher on the irrigated plot than on the non-irrigated plot. With progressive increases in soil salinity, sodicity, and pH in the upper soil layers of the irrigated plot, several herbaceous species could no longer be detected, while L. alyssoides became dominant. This supports the possibility of interspecific variation among the herbaceous species in soil-related stress resistance, and thus variation within the species pool to effectively serve in wastewater treatment processes.
Application of our findings on L. alyssoides to semiarid land management potentially has both positive and negative ramifications. Just as this species provided ground cover and wastewater attenuating abilities on the irrigated intershrub spaces, it appeared to become invasive, and species diversity declined. This may affect land reclamation processes after cessation of land application to such a site. Vance et al. (2008) arrived at the same conclusion concerning the difficulty of land reclamation after Wyoming land application sites receive saline–sodic irrigation waters. In that study, the overall species diversity declined as several non-native species expressed high salinity and sodicity tolerance, produced high biomass, and replaced native perennial species.
Cox et al. (2006) suggested that "nonresource factors," such as soil salinity and pH, are important determinants of desert plant species richness, while on the other hand, it is argued that soil N availability may determine desert vegetation community species richness (Brooks, 2003). Thus, in the Vance et al. (2008) study noted previously, the authors posed the question as to whether the increased biomass by the non-native species resulted from increased competitive ability in response to increasing water supply, or to higher tolerance to increased soil salinity, sodicity, and pH. Similarly in our study, the relative importance of soil water, NO 3 –-N, Olsen-P, soluble K +, salinity, alkalinity, and sodicity in stimulating L. alyssoides , cannot be determined with certainty. Grace (2001) stated that in natural environments, plant species that tolerate high levels of soil salinity may be good for community biomass, which supports the nonresource factor and species pools hypotheses for biomass stimulation of L. alyssoides , and ecological value of such aggressive indigenous species to Chihuahuan Desert lands receiving saline–sodic irrigation waters.
Conclusions and Recommendations
True replications (i.e., blocking) and wide variability in wastewater application rate and quality over time were obvious limitations in this study. However, these limitations are expected when conducting research under real situations with operational WWTPs and with inherently high variation in wastewater flow and quality (Britz et al., 2006). Obvious differences in soil chemical properties between irrigated and non-irrigated plots were observed and discussed. Soil analysis accounted for most of the mineral deposits; the expected increases in soil salinity, sodicity, and pH; and the increases in soil nutrients that support beneficial reuse of wastewater for vegetation. Clearly, site-to-site variation in shrub density must be considered in the design and operation of land application systems in the Chihuahuan Desert.
A "cascade" of events following minimization of influent Cl - in 2003 had profound and systemic effects, which included i) Ca 2+ precipitation and sedimentation in the lagoon; ii) loss in effluent Ca 2+; iii) increases in effluent total salinity, sodicity, and alkalinity; iv) increases in soil saturation extract salinity, sodicity, and pH below all three of the ground types; and v) changes in the vegetation. These outcomes demonstrate the challenges associated with balancing Cl – discharge regulations on the one hand with maintenance of the lagoons and protection of the soil and vegetation on the other, particularly in semiarid regions where the soils are already alkaline and, in our case, the influent supply already had an SAR of 10–13. There is a need for industrial processing alternatives to Cl –-containing chemical compounds that pose a minimal sodicity and alkalinity risk to soils and vegetation, and that prevent Ca 2+ sedimentation in settling basins.
Potential for land application of treated industrial effluent in New Mexico to mitigate Rio Grande salinization is appropriate for long-term needs of the region (Miyamoto et al., 1995). The present study was confronted by the reality of water scarcity in the Chihuahuan Desert in that the effluent supply limitations prevented full utilization of the site and determination of the maximum wastewater processing potential of the soil and vegetation. The slow-rate land application altered the soil in ways that not only increased soil N, P, and K + fertility, but that also increased vegetation growth-suppressive factors (EC e, SAR e, pH, and Cl -), the latter up to high levels in agricultural terms (Ayers and Westcott, 1985). Even though vegetation growth was stimulated under the conditions of this study and no NO 3 –-N leaching losses occurred below 2 m, higher effluent irrigation rates could increase the risks of NO 3 –-N losses and physiological stress to the vegetation. Further research could resolve these uncertainties for similar effluent qualities and environmental conditions.
Chihuahuan Desert vegetation was stimulated by nutrient-containing treated effluent despite increased soil salinity, sodicity, and pH. While our vegetation mineral recovery data are only approximate, we are unaware of similar land application studies involving native vegetation. Our estimates of vegetation mineral recovery show the extent to which treated wastewater contaminants are being utilized and not simply moved from one place to another. This is an important consideration in complying with the pollution prevention permit that, prior to this study, has not been addressed in New Mexico. The aboveground vegetation recovered meaningful amounts of effluent-applied N and Ca 2+, but was not effective in recovering effluent P, K +, Mg 2+, Na +, and Cl -. High leaf Na + and Cl - accumulation by the shrubs and limited Na + and Cl - recovery by the combined vegetation raise concern over sustainability of Chihuahuan Desert rangelands receiving continuous irrigation with saline–sodic industrial effluent.
Lepidium alyssoides produced the most biomass of all species and may be of value in desert land application systems that release high-Na + effluent. This species is not regarded as a noxious weed in New Mexico, at least under natural rangeland conditions (Allred, 2008). However, it appears to be invasive under induced conditions of high sodicity. If the objective is to maximize biomass and effluent mineral uptake by aggressive, natrophilic species, high-Na + discharge may be desirable. If the objective is to preserve the shrubland community and maintain diversity within the intershrub spaces, then placing discharge limits on Na + may be a preferred option.
This study can serve as a foundation for studying long-term viability of Chihuahuan Desert vegetation communities exposed to saline–sodic effluent. We hope that our pioneering efforts will encourage others to make improvements upon the design and analysis of semiarid wastewater land application experiments.
References
Adhikari P., M.K. Shukla, J.G. Mexal, and P. Sharma. 2011. Assessment of the soil physical and chemical properties of desert soils irrigated with treated wastewater using principal component analysis. Soil Science, 176, 356–366.
Aguiar, M.R., and O.E. Sala. 1999. Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends in Ecology and Evolution, 14, 273–277.
Al-Jibury, L.K. 1972. Salt tolerance of some desert shrubs in relation to their distribution in the southern desert of North America [Ph.D. dissertation]. Tempe: Arizona State University.
Allen, A.P., W.T. Pockman, C. Restrepo, and B.T. Milne. 2008. Allometry, growth and population regulation of the desert shrub Larrea tridentata. Functional Ecology, 22, 197–204.
Allred, K.W. 2008. Flora neomexicana I: The vascular plants of New Mexico. Lulu.com.
American Public Health Association, American Water Works Association, and Water Environment Federation. 1992. Standard methods for the examination of water and wastewater, 18th ed. Washington D.C.
American Public Health Association, American Water Works Association, and Water Environment Federation. 1995. Standard methods for the examination of water and wastewater, 19th ed. Washington, D.C.
American Public Health Association, American Water Works Association, and Water Environment Federation. 1998. Standard methods for the examination of water and wastewater, 20th ed. Washington D.C.
Anning, D.W., N.J. Bauch, S.J. Gerner, M.E. Flynn, S.N. Hamlin, S.J. Moore, D.H. Schaefer, S.K. Anderholm, and L.E. Spangler. 2007. Dissolved solids in basin-fill aquifers and streams in the southwestern United States [Scientific Investigations Report 2006–5315]. Reston, VA: U.S. Geological Survey.
Ayers, R.S., and D.W. Westcot. 1985. Water quality for agriculture [Irrigation and Drainage Paper 29]. Rome: Food and Agricultural Organization of the United Nations.
Babcock, M., M.K. Shukla, G.A. Picchioni, J.G. Mexal, and D. Daniel. 2009. Chemical and physical properties of Chihuahuan Desert soils irrigated with industrial effluent. Arid Land Research and Management, 23, 47–66.
Bachelet, D., W.M. Jarrell, and R.A. Virginia. 1986. Simulation model of a laboratory-grown phreatophytic woody legume. Tree Physiology, 2, 205–214.
Barker, D.H., C. Vanier, E. Naumburg, T.N. Charlet, K.M. Nielsen, B.A. Newingham, and S.D. Smith. 2006. Enhanced monsoon precipitation and nitrogen deposition affect leaf traits and photosynthesis differently in spring and summer in the desert shrub Larrea tridentata. New Phytologist, 169, 799–808.
Barton, L., L.A. Schipper, G.F. Barkle, M. McLeod, T.W. Speir, M.D. Taylor, A.C. McGill, A.P. van Schaik, N.B. Fitzgerald, and S.P. Pandey. 2005. Land application of domestic effluent onto four soil types: Plant uptake and nutrient leaching. Journal of Environmental Quality, 34, 635–643.
Bastian, R.K. 2005. Interpreting science in the real world for sustainable land application. Journal of Environmental Quality, 34, 174–183.
Bernstein, L. 1980. Salt tolerance of fruit crops [USDA Agriculture Information Bulletin No. 292]. Washington, D.C.: U.S. Department of Agriculture.
Biggar, J.W., D.E. Rolston, and D.R. Nielsen. 1984. Transport of salts by water. California Agriculture, 38(10), 10–11.
Bremner, J.M. 1996. Total nitrogen. In D.L. Sparks, A.L. Page, P.A. Heimke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C.T. Johnston, and M.E. Sumner (Eds.), Methods of soil analysis, part 3, chemical methods (pp. 1085–1121). Madison, WI: Soil Science Society of America.
Brenton, C.M., E.B. Fish, and R. Mata-González. 2007. Macronutrient and trace element leaching following biosolids application on semiarid rangeland soils. Arid Land Research and Management, 21, 143–156.
Bridgham, D.O., W.A. Britton, B.A. Patrie, and J.A. Lawson. 1977. Land application of food processing wastewater. In R.C. Loehr (Ed.), Food, fertilizer, and agricultural residues (pp. 67–77), Proceedings of the 1977 Cornell Agricultural Waste Management Conference, Ann Arbor Science, Inc., Ann Arbor, MI.
Britz, T.J., C. van Schalkwyk, and Y.T. Hung. 2006. Treatment of dairy processing wastewaters. In L.K. Wang, Y.T. Hung, H.H. Lo, and C. Yapijakis (Eds.), Waste treatment in the food processing industry (pp. 1–28). New York: Taylor and Francis Group.
Brooks, M.L. 2003. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. Journal of Applied Ecology, 40, 344–353.
Bulloch, H.E., and R.E. Neher. 1980. Soil survey of Doña Ana county area, New Mexico. Washington, D.C.: U.S. Department of Agriculture, Soil Conservation Service.
Buonopane, M., L.F. Huenneke, and M. Remmenga. 2005. Community response to removals of plant functional groups and species from a Chihuahuan Desert shrubland. Oikos, 110, 67–80.
Causin, H.F., D.C. Tremmel, T.W. Rufty, and J.F. Reynolds. 2004. Growth, nitrogen uptake, and metabolism in two semiarid shrubs grown at ambient and elevated atmospheric CO 2 concentrations: Effects of nitrogen supply and source. American Journal of Botany, 91, 565–572.
Cox, S.B., C.P. Bloch, R.D. Stevens, and L.F. Huenneke. 2006. Productivity and species richness in an arid ecosystem: A long-term perspective. Plant Ecology, 186, 1–12.
Craig, J.E., and S.R. Abella. 2008. Vegetation of grassy remnants in the Las Vegas Valley, southern Nevada. Desert Plants, 24(1), 16–23.
Cross, A.F., and W.H. Schlesinger. 1999. Plant regulation of soil nutrient distribution in the northern Chihuahuan Desert. Plant Ecology, 145, 11–25.
Doremus, D. 2008. Rio Grande salinity management–A real possibility? Southwest Hydrology, 7(2), 24–25.
Ettershank, G., J. Ettershank, M. Bryant, and W.G. Whitford. 1978. Effects of nitrogen fertilization on primary production in a Chihuahuan Desert ecosystem. Journal of Arid Environments, 1, 135–139.
Faris, B. 2009. Nitrate groundwater issues: New Mexico's perspective. Southwest Hydrology, 8(4), 26–27.
Felker, P., P.R. Clark, A.E. Laag, and P.F. Pratt. 1981. Salinity tolerance of the tree legumes: Mesquite ( Prosopis glandulosa var. Torreyana, P. velutina, and P. articulata), algarrobo ( P. chilensis), kiawe ( P. pallida), and tamarugo ( P. tamarugo) grown in sand culture on nitrogen-free media. Plant and Soil, 61, 311–317.
Fisher, F.M., J.C. Zak, G.L. Cunningham, and W.G. Whitford. 1988. Water and nitrogen effects on growth and allocation patterns of creosotebush in the northern Chihuahuan Desert. Journal of Range Management, 41, 387–391.
Flowers, T.J., and A. Läuchli. 1983. Sodium versus potassium: Substitution and compartmentation. In A. Läuchlii and R.L. Bieleski (Eds.), Encyclopedia of plant physiology, vol. 15B, Inorganic plant nutrition (pp. 651–681). New York: Springer-Verlag.
Francis, A., and S.I. Warwick. 2007. The biology of invasive alien plants in Canada. 8. Lepidium latifolium L. Canadian Journal of Plant Science, 87, 639–658.
Frankenberger, W.T., M.A. Tabatabai, D.A. Adriano, and H.E. Doner. 1996. Bromine, chlorine, and fluorine. In D.L. Sparks, A.L. Page, P.A. Heimke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C.T. Johnston, and M.E. Sumner (Eds.), Methods of soil analysis, part 3, chemical methods (pp. 833–867). Madison, WI: Soil Science Society of America.
Ganjegunte, G.K., L.A. King, and G.F. Vance. 2008. Cumulative soil chemistry changes from land application of saline–sodic waters. Journal of Environmental Quality, 37, S-128–S-238.
Gavlak, R.G., D.A. Horneck, and R.O. Miller 1994. Plant, soil, and water reference methods for the western region [Western Coordinating Committee, Western Region Extension Publication 125]. Corvallis, OR: Western Rural Development Center.
Ghaly, A.E., D.G. Rushton, and N.S. Mahmoud. 2007. Potential air and groundwater pollution from continuous high land application of cheese whey. American Journal of Applied Sciences, 4, 619–627.
Gibbens, R.P., and J.M. Lenz. 2001. Root systems of some Chihuahuan Desert plants. Journal of Arid Environments, 49, 221–263.
Gibbens, R.P., R.P. McNeely, K.M. Havstad, R.F. Beck, and B. Nolen. 2005. Vegetation changes in the Jornada basin from 1858 to 1998. Journal of Arid Environments, 61, 651–668.
Gile, L.H., J.W. Hawley, and R.B. Grossman. 1981. Soils and geomorphology in the basin and range area of southern New Mexico–Guidebook to the desert project [Memoir 39]. Socorro, NM: New Mexico Bureau of Mines and Mineral Resources.
Grace, J.B. 2001. The roles of community biomass and species pools in the regulation of plant diversity. Oikos, 92, 193–207.
Gutierrez, J.R., and W.G. Whitford. 1987. Chihuahuan Desert annuals: Importance of water and nitrogen. Ecology, 68, 2032–2045.
Greenway, H., and R. Munns. 1980. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology, 31, 149–190.
Halvorson, G.A., and K.J. Lang. 1989. Revegetation of a salt water blowout site. Journal of Range Management, 42, 61–65.
Hamerlynck, E.P., T.E. Huxman, J.R. McAuliffe, and S.D. Smith. 2004. Carbon isotope discrimination and foliar nutrient status of Larrea tridentata (creosote bush) in contrasting Mojave Desert soils. Oecologia, 138, 210–215.
Huenneke, L.F., D. Clason, and E. Muldavin. 2001. Spatial heterogeneity in Chihuahuan Desert vegetation: Implications for sampling methods in semi-arid ecosystems. Journal of Arid Environments, 47, 257–270.
Huenneke, L.F., J.P. Anderson, M. Remmenga, and W.H. Schlesinger. 2002. Desertification alters patterns of aboveground net primary production in Chihuahuan ecosystems. Global Change Biology, 8, 247–264.
Hughes, R.F., S.R. Archer, G.P. Asner, C.A. Wessman, C. McMurtry, J. Nelson, and R.J. Ansley. 2006. Changes in aboveground primary production and carbon and nitrogen pools accompanying woody plant encroachment in a temperate savanna. Global Change Biology, 12, 1733–1747.
International Boundary and Water Commission. 2003. Flow of the Rio Grande and related data [Water Bulletin 73, online]. Retrieved June 14, 2011, from http://www.ibwc.state.gov/Water_Data/water_bulletins.html
International Boundary and Water Commission. 2004. Flow of the Rio Grande and related data [Water Bulletin 74, online]. Retrieved June 14, 2011, from http://www.ibwc.state.gov/Water_Data/water_bulletins.html
James, J.J., R.L. Tiller, and J.H. Richards. 2005. Multiple resources limit plant growth and function in a saline-alkali desert community. Journal of Ecology, 93, 113–126.
Jarrell, W.M., and R.A. Virginia. 1984. Salt tolerance of mesquite. California Agriculture, 38(10), 28.
Jarrell, W.M., and R.A. Virginia. 1990a. Response of mesquite to nitrate and salinity in a simulated phreatic environment: Water use, dry matter, and mineral nutrient accumulation. Plant and Soil, 125, 185–196.
Jarrell, W.M., and R.A. Virginia. 1990b. Soil cation accumulation in a mesquite woodland: Sustained production and long-term estimates of water use and nitrogen fixation. Journal of Arid Environments, 13, 51–53.
Jones, J.B., Jr., B. Wolf, and H.A. Mills. 1991. Plant analysis handbook 1. Methods of plant analysis and interpretation. Athens, GA: Micro Macro International.
Khumalo, G., J. Holechek, M. Thomas, and F. Molinar. 2008. Soil depth and climate effects on desert vegetation dynamics. Rangeland Ecology and Management, 61, 269–274.
Lajtha, K. 1987. Nutrient reabsorption efficiency and the response to phosphorus fertilization in the desert shrub Larrea tridentata (DC.) Cov. Biogeochemistry, 4, 265–276.
Lajtha, K., and M. Klein. 1988. The effect of varying nitrogen and phosphorus availability on nutrient use by Larrea tridentata, a desert evergreen shrub. Oecologia, 75, 348–353.
Lajtha, K., and W.G. Whitford. 1989. The effect of water and nitrogen amendments on photosynthesis, leaf demography, and resource-use efficiency in Larrea tridentata, a desert evergreen shrub. Oecologia, 80, 341–348.
Levings, G.W., D.F. Healy, S.F. Richey, and L.F. Carter. 1998. Water quality in the Rio Grande Valley, Colorado, New Mexico, and Texas, 1992-95 [Circular 1162]. Reston, VA: U.S. Geological Survey.
Liu, Y.Y., and R.J. Haynes. 2010. Long-term irrigation with dairy factory wastewater influences soil quality. World Academy of Science, Engineering and Technology, 70, 577–581.
MacKay, W.P., F.M. Fisher, S. Silva, and W.G. Whitford. 1987. The effects of nitrogen, water and sulfur amendments on surface litter decomposition in the Chihuahuan Desert. Journal of Arid Environments, 12, 223–232.
Maestre, F.T., and J.F. Reynolds. 2006. Small-scale spatial heterogeneity in the vertical distribution of soil nutrients has limited effects on the growth and development of Prosopis glandulosa seedlings. Plant Ecology, 183, 65–75.
McArthur, E.D., and S.G. Kitchen. 2007. Shrubland ecosystems: Importance, distinguishing characteristics, and dynamics. In R.E. Sosebee, D.B. Webster, C.M. Britton, E.D. McArthur, and S.G. Kitchen (Eds.), Proceedings: Shrubland dynamics–fire and water, Fort Collins, CO, August 10–12, 2004 [RMRS–P–47] (pp. 3–10). Ft. Collins, CO: U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station.
McAuliffe, J.R., E.P. Hamerlynck, and M.C. Eppes. 2007. Landscape dynamics fostering the development and persistence of long-lived creosotebush ( Larrea tridentata) clones in the Mojave Desert. Journal of Arid Environments, 69, 96–126.
Miller, R.E., and L.F. Huenneke. 2000a. The relationship between density and demographic variation within a population of Larrea tridentata. Southwestern Naturalist, 45, 313–321.
Miller, R.E., and L.F. Huenneke. 2000b. Demographic variation in a desert shrub, Larrea tridentata, in response to a thinning treatment. Journal of Arid Environments, 45, 315–323.
Miller, G.R., Y. Rubin, K.U. Mayer, and P.H. Benito. 2008. Modeling vadose zone processes during land application of food processing waste water in California's central valley. Journal of Environmental Quality, 37, S-43–S-57.
Miyamoto, S., G.R. Gobran, and K. Piela. 1985. Salt effects on seedling growth and ion uptake of three pecan rootstock cultivars. Agronomy Journal, 77, 383–388.
Miyamoto, S., L.B. Fenn, and D. Swietlik. 1995. Flow, salts, and trace elements in the Rio Grande: A review [Publication TR-169]. College Station, TX: Texas Water Resources Institute.
Molinar, F., J. Holechek, D. Galt, and M. Thomas. 2002. Soil depth effects on Chihuahuan Desert vegetation. Western North American Naturalist, 62, 300–306.
Mulvaney, R.L. 1996. Nitrogen–Inorganic forms. In D.L. Sparks, A.L. Page, P.A. Heimke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C.T. Johnston, and M.E. Sumner (Eds.), Methods of soil analysis, part 3, chemical methods (pp. 1123–1184). Madison, WI: Soil Science Society of America.
Natural Resources Conservation Service. 2012a. Plants database [Online]. Retrieved May 24, 2012, from http://plants.usda.gov
Natural Resources Conservation Service. 2012b. Basin-wide reservoir summary [Online]. Retrieved July 10, 2012, from http://www.wcc.nrcs.usda.gov/ftpref/data/water/basin_reports/new_mexico/wy2012/barenm6.txt
Nelson, D.W., and L.E. Sommers. 1996. Total carbon, inorganic carbon, and organic matter. In D.L. Sparks, A.L. Page, P.A. Heimke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C.T. Johnston, and M.E. Sumner (Eds.), Methods of soil analysis, part 3, chemical methods (pp. 961–1010). Madison, WI: Soil Science Society of America.
New Mexico Climate Center. 2011. Index of stations with pan evaporation data [Online]. Retrieved June 14, 2011, from http://weather.nmsu.edu/Pan_Evaporation/state_evap.htm
Nilsen, E.T., P.W. Rundel, and R.M Sharifi. 1981. Summer water relations of the desert phreatophyte Prosopis glandulosa in the Sonoran Desert of southern California. Oecologia, 50, 271–276.
Nilsen, E.T., R.A. Virginia, and W.M. Jarrell. 1986. Water relations and growth characteristics of Prosopis glandulosa var. torreyana in a simulated phreatophytic environment. American Journal of Botany, 73, 427–433.
O'Connor, G.A., H.A. Elliott, N.T. Basta, R.K. Bastian, G.M. Pierzynski, R.C. Sims, and J.E. Smith, Jr. 2005. Sustainable land application: An overview. Journal of Environmental Quality, 34, 7–17.
Olsen, S.R., C.V. Cole, F.S. Watanabe, and L.A. Dean. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate [Circular 939]. Washington, D.C.: U.S. Department of Agriculture.
Perkins, S.R., and M.K. Owens. 2003. Growth and biomass allocation of shrub and grass seedlings in response to predicted changes in precipitation seasonality. Plant Ecology, 168, 107–120.
Picchioni, G.A, S. Miyamoto, and J.B. Storey. 1990. Salt effects on growth and ion uptake of pistachio rootstocks. Journal of the American Society for Horticultural Science, 115, 647–653.
Picchioni, G.A., S. Miyamoto, and J.B. Storey. 1991. Rapid testing of salinity effects on pistachio rootstock seedlings. Journal of the American Society for Horticultural Science, 116, 555–559.
Picchioni, G.A., P.H. Brown, S.A. Weinbaum, and T.T. Muraoka. 1997. Macronutrient allocation to leaves and fruit of mature, alternate bearing pistachio trees: Magnitude and seasonal patterns at the whole canopy level. Journal of the American Society for Horticultural Science, 122, 267–274.
Picchioni, G.A. and C.J. Graham. 2001. Salinity, growth, and ion uptake selectivity of container-grown Crataegus opaca. Scientia Horticulturae, 90, 151–166.
Picchioni, G.A., M.K. Shukla, J.G. Mexal, M. Babcock, A. Ruiz, T.W. Sammis, and D.S. Rodriguez. 2012a. Land application of treated industrial wastewater on a Chihuahuan Desert Shrubland: Implications for water quality and mineral deposition. Arid Land Research and Management, 26, 211–226.
Picchioni, G.A., J.G. Mexal, M.K. Shukla, A. Ruiz, M. Babcock, D.L. Daniel, and D.S. Rodriguez. 2012b. Land application of treated industrial wastewater on a Chihuahuan Desert Shrubland: Impacts on the natural vegetation. Arid Land Research and Management, 26, 312–327.
Reynolds, J.F., R.A. Virginia, P.R. Kemp, A.G. de Soyza, and D.C. Tremmel. 1999. Impact of drought on desert shrubs: Effects of seasonality and degree of resource island development. Ecological Monographs, 69, 69–106.
Rhoades, J.D. 1996. Salinity: Electrical conductivity and total dissolved solids. In D.L. Sparks, A.L. Page, P.A. Heimke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C.T. Johnston, and M.E. Sumner (Eds.), Methods of soil analysis, part 3, chemical methods (pp. 417–435). Madison, WI: Soil Science Society of America.
Ruiz, A., T.W. Sammis, G.A. Picchioni, J.G. Mexal, and W.A. Mackay. 2006. An irrigation scheduling protocol for treated industrial effluent in the Chihuahuan Desert. Journal–American Water Works Association, 98, 122–133.
Sammis, T.W., C.L. Mapel, D.G. Lugg, R.R. Lansford, and J.T. McGuckin. 1985. Evapotranspiration crop coefficients predicted using growing-degree-days. Transactions–American Society of Agricultural Engineers, 28, 773–780.
Sandquist, D.R., W.S.F. Schuster, L.A. Donovan, S.L. Phillips, and J.R. Ehleringer. 1993. Differences in carbon isotope discrimination between seedlings and adults of southwestern desert perennial plants. Southwestern Naturalist, 38, 212–217.
Schlesinger, W.H., J.A. Raikes, A.E. Hartley, and A.F. Cross. 1996. On the spatial pattern of soil nutrients in desert ecosystems . Ecology, 77, 364–374.
Sharifi, M.R., F.C. Meinzer, P.W. Rundel, and E.T. Nilsen. 1990. Effect of manipulating soil water and nitrogen regimes on clipping production and water relations of creosote bush [General Technical Report, Intermountain Research Station, 276, 245–249]. Washington, D.C.: USDA Forest Service.
Smith, P.F. 1976. Collapse of 'Murcott' tangerine trees. Journal of the American Society for Horticultural Science, 101, 23–25.
Snedecor, G.W., and W.G. Cochran. 1989. Statistical methods, 8th ed. Ames, IA: Iowa State University Press.
Sparks, D. 1977. Effects of fruiting on scorch, premature defoliation, and nutrient status of 'Chickasaw' pecan leaves. Journal of the American Society for Horticultural Science, 102, 669–673.
Teague, W.R., R.J. Ansley, W.E. Pinchak, S.L. Dowhower, S.A. Gerrard, and J.A. Waggoner. 2008. Interannual herbaceous biomass response to increasing honey mesquite cover on two soils. Rangeland Ecology and Management, 61, 496–508.
United States Environmental Protection Agency. 1979. Methods for chemical analysis of water and wastes [EPA-600/79-020]. Cincinnati, OH: U.S. Environmental Protection Agency, Environmental Monitoring Systems Laboratory.
United States Environmental Protection Agency. 1982. Handbook for sampling and sample preservation of water and wastewater [PB83-124503]. Cincinnati, OH: U.S. Environmental Protection Agency, Environmental Monitoring Systems Laboratory.
United States Environmental Protection Agency. 1994. Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry [Method 200.7. Revision 4.4]. Cincinnati, OH: U.S. Environmental Protection Agency, Environmental Monitoring Systems Laboratory.
United States Environmental Protection Agency. 1997. Microwave assisted acid digestion of sediments, sludges, soils, and oils. Methods for evaluating solid waste, physical/chemical properties [SW-846; CD ROM, Version 2]. Alexandria, VA: National Technical Information Service.
United States Salinity Laboratory Staff. 1954. Diagnosis and improvement of saline and alkali soils [Handbook 60]. Washington D.C.: U.S. Department of Agriculture.
Vance, G.F., L.A. King, and G.K. Ganjegunte. 2008. Soil and plant responses from land application of saline-sodic waters: Implication of management. Journal of Environmental Quality, 37, S-139–S-148.
Wiebe, H.H., and H. Walter. 1972. Mineral ion composition of halophytic species from northern Utah. American Midland Naturalist, 86, 241–245.
Wendorff, W.L., and S. Matzke. 1993. Phosphorus in major varieties of whey produced in Wisconsin. Dairy, Food and Environmental Sanitation, 13, 166–167.
Wester, D.B. 1992. Viewpoint: Replication, randomization, and statistics in range research. Journal of Range Management, 45, 285–290.
Acknowledgments
This study was supported by the New Mexico State University Agricultural Experiment Station, Southwest Consortium for Environmental Research and Policy, and USDA Cooperative State Research, Education, and Extension Service–Rio Grande Basin Initiative Grants. We thank Dan Santantonio, Mark Rodriguez, Eric Lopez, Des Stewart, Roman Garcia, Gilbert Morales, Doug Paczynski, Raymond Parson, and Jose Hernandez of the City of Las Cruces Utilities Division for performing wastewater treatment plant and irrigation system operations. For sampling and sample processing, we thank former New Mexico State University (NMSU) graduate students Michael Babcock, Alejandro Ruiz, Denise Rodriguez, and Michaela Mattes, and Universidad Autónoma de Ciudad Juárez undergraduate interns Dulse Janet Chávez Fernández, Aldo Raúl Piñón Villarreal, Adelaido Ángel Hernández, and Jonathan Campaña Lozoya. We also thank Dr. Kelly Allred (NMSU Department of Animal and Range Science) for providing identifications of the herbaceous plant species on the experimental plots, and Dr. David Daniel (NMSU Department of Economics and International Business) for performing the chi-square analyses of the shrub populations.
Abbreviations
ANPP, aboveground net primary production; APHA, American Public Health Association; BOD, biological oxygen demand; COD, chemical oxygen demand; cv, coefficient of variation; EC, electrical conductivity; EC e, electrical conductivity of the soil saturation extract; ET, evapotranspiration; IBWC, Interstate Boundary and Water Commission; ICP-ES, inductively-coupled plasma emission spectrometry; NRCS, Natural Resources Conservation Service; NMED-GWQB, New Mexico Environment Department Groundwater Quality Bureau; PCA, projected canopy area; R 2, coefficient of determination; SAR, sodium adsorption ratio; SAR e, sodium adsorption ratio of the soil saturation extract; TDA, total dissolved anions; TDC, total dissolved cations; TDM, total dry matter; TDS, total dissolved solids; TKN, total Kjeldahl-nitrogen; TSS, total suspended solids; USEPA, United States Environmental Protection Agency; WMIP, West Mesa Industrial Park; WWTP, wastewater treatment plant.

Geno A. Picchionio is a professor of horticulture in the Department of Plant and Environmental Sciences at New Mexico State University. He earned his Ph.D. at Texas A&M University. His research and teaching efforts focus on plant salinity stress, transport processes in plants, inorganic plant nutrition, plant membrane function, plant mineral nutrition, and greenhouse management.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at aces.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
January 2014, Las Cruces, NM


