Chile Seed Germination as Affected by Temperature and Salinity1
New Mexico Chile Task Force Report 2
Robert Flynn, Richard Phillips, April Ulery, Richard Kochevar, Linda Liess and Magdalena Villa, State Seed Analyst, New Mexico Department of Agriculture
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Associate Professor and Extension Agronomy and Soils Specialist, Cooperative Extension Service, Project Manager, Cooperative Extension Servic, Assistant Professor, Soils, Department of Agronomy and Horticulture, State Seed Analyst, New Mexico Department of Agriculture, Research Specialist, Extension Plant Sciences, Student, Department of Agronomy and Horticulture.
Research Summary
For economic reasons, chile is planted early, which often exposes the seed to adverse conditions that affect stand establishment. High levels of soil salinity, common in many areas of the Southwest, also influence stand establishment, since chile is particularly sensitive to salt levels. This laboratory study evaluated how temperature and salinity affect seed germination. Four chile seed types (fresh green, fresh jalapeño, dry red, and fresh red) were subjected to incubation temperatures of 10°C (50°F), 15°C (59°F), and 20°C (68°F) with varying levels of saline water during germination. The saline levels were 1, 3, 5, 7, and 9 deciSiemens per meter (dS/m). All chile types also were tested using standard germination protocols, with the salt content of the wetting solution remaining constant. Germination was evaluated 6, 9, 12, 15, 18, and 21 days after placement in the incubators. Seeds incubated at 10°C (50°F) did not germinate. At an incubation temperature of 15oC (59°F), less-than-optimal germination occurred during the 21-day test. After only 12 days, all chile types tested at 20oC (68°F) came to within 90% of maximum germination. Salinity levels at or above 7 dS/m reduced germination by as much as 19% under 20°C (68°F) conditions and by as much as 27% under 15°C (59°F) conditions. Extrapolating these results to field conditions suggests that chile should be planted into a seedbed with salinity less than 3 dS/m and an average temperature of 20°C (68°F) for 12 days. Although salinity may have a relatively small impact on final germination, it can still have a major impact on stand establishment and, ultimately, on yield. Furthermore, there was a 13% difference in germination among varieties under standard conditions. That variation should be investigated further to improve consistent germination for all chile seed.
Background
Uniform stand establishment for chile pepper production is essential to maintaining profitable yields. A 1999 survey of commercial chile fields in southern New Mexico indicated that as many as 64% of the farms started the season with poorly stocked chile stands (Phillips, unpublished data, 2000). Poor stands are a result of many factors, including seed quality; environment; water and soil quality; site preparation; plant uniformity; irrigation practices; and other management practices. This study focused on two critical environmental factors: temperature and salinity. By the very nature of the commercial chile industry in New Mexico, these two environmental factors always will offset the uniformity of chile stand establishment. Learning to manage them is a crucial factor in producing profitable yields.
Processor perspective
The balance between processing capacity and the length of the chile growing season dictates the timing of planting and harvesting. Processors need to spread the harvest out to match their processing capacity. To maximize their use of capital investment in equipment, processors contract the flow of raw materials over as long a period as possible. Growers’ decisions about planting dates are controlled by two factors: the processors’ need to balance capacity and product quality, and supply and demand. Fresh green chile, jalapeños, and fresh red chile have a very short storage life before processing, so processors must move the raw material quickly. Red chile is a more stable product, but it, too, loses quality with time. It is important that processing plants begin processing early and continue throughout a maximum harvest window.
Supply and demand affect the time of planting, since growers realize that raw materials are in short supply during the processing plant start-up phase. Historically, if the farmer can deliver chile early, the demand is unlimited. This is in contrast to midseason circumstances, when the processing plants are full and growers are forced to slow down delivery of raw material. These two factors -- processing capacity and the growers’ willingness to gamble on early delivery -- result in many acres of chile being planted into a growing environment with less-than-optimal biophysical requirements.
Environmental perspective
Soil temperature. While spring weather in the southwestern United States practically begs farmers to start planting, it is soil, not air, temperature that governs seed germination. Some sources give minimum temperatures for germination. These are the lowest temperatures at which germination will occur for specific crops. The length of time for germination will be much longer at less-than-optimal temperatures. Planting at lower temperatures also results in greatly reduced germination.
Some crops require fluctuating temperatures on a daily or seasonal basis. In other crops, germination is affected by the interrelation of temperature and light. For example, celery requires temperatures below 50°F to germinate, if it is held in the dark at a constant temperature. In diffused light, however, it will germinate at 70°F; and with a 10-degree day/night fluctuation, it will germinate at an 85°F day temperature. A review of the chile seed germination literature (Bosland and Votava, 2000) indicates that day/night temperature fluctuations of 30°/15° C (86°/59°F) at 16 hours of light and 8 hours of darkness is biologically the best temperature and lighting range for chile germination.
Salinity. Soil salinity, if not properly managed, can become a limiting factor for chile stand establishment. Excess salinity within the plant root zone has a deleterious effect on plant growth. Tanksley and Phillips (1985) reported a decline in seed germination for the red chile variety, ‘NuMex R Naky’, at 7.8 and 9.4 dS/m. Growth suppression usually is initiated at some threshold value of salinity that varies with crop tolerance and external environmental factors. Plants generally are tolerant during germination but become more sensitive during the emergence and early seedling growth stages (Rhoades et al., 1992). It is imperative to keep salinity low in the seedbed during germination and stand establishment. It also is important to realize that salt tolerance data cannot provide accurate, quantitative crop yield losses from salinity for every situation, since actual responses to salinity vary with other conditions, including agronomic factors, soil and water management, climate, crop variety, and growth stage.
Crop choice perspective. Crop variety can have a profound effect on stand establishment under different environmental conditions. Since chile is an important rotational crop in the Southwest, a brief overview of chile types could help explain differences that arise when considering what varieties to plant under less-than-optimal conditions.
The place of origin for all commercial chile peppers grown in the Southwest is Latin America (DeWitt and Bosland, 1996). While production has been adapted to temperate climates by growing chile as an annual plant, it originated in a frost-free climate and grew as a perennial (Bosland et al., 1996). There are four major chile types grown in New Mexico. They include fresh green, jalapeño, dry red (paprika), and red cayenne. How each of these commercial chile types responds to soil temperature and salinity conditions is not well understood. Therefore, a replicated study conducted under controlled environmental conditions was performed to observe the interaction of these two critical environmental factors.
Hypothesis
The hypothesis of this study was that low temperatures coupled with high salinity would negatively affect chile seed germination.
Materials and Methods
The New Mexico Department of Agriculture’s Seed Testing Laboratory, approved by the Association of Official Seed Analysts (AOSA), conducted the germination tests in conjunction with research associates of New Mexico State University’s Chile Task Force.
Twenty treatments, consisting of five salinity levels for each of four chile types with four replications, were arranged in a completely randomized design within each temperature regime (10°C, 15°C, and 20°C). Time was considered a split plot with multiple observation times (6, 9, 12, 15, 18, and 21 days after placement). Salinity levels were 1, 3, 5, 7, and 9 dS/m. There were four types of chile seed used: ‘AZ-20’ (a fresh green chile); ‘Sonora’ (a dry, red paprika type); ‘TAM’ (a jalapeño type for fresh market); and ‘Large Red Thick’ (a cayenne type). The experimental unit was 100 seeds placed on standard AOSA-approved seed germination blotter paper. Seeds were randomly chosen for each treatment without regard to any physical characteristics or seed viability. A total of 6,400 seeds were tested.
The AOSA standard germination test for pepper (Capsicum sp.) was used as a control or standard for comparison to the imposed incubation temperatures. The standard seed test was run at 20°C (68°F) in 16 hours of darkness followed by 30°C (86°F) for 8 hours of light and used distilled water (electrical conductivity < 0.1 dS/m) to saturate the blotter and moisten the seeds. Saline solutions were prepared from a mixture of sodium and calcium chloride reagents in a 1:1 equivalent ratio to attain electrical conductivities of 1, 3, 5, 7, and 9 dS/m. The amount of each chloride salt in the test solutions was determined graphically from U.S. Salinity Laboratory Handbook 60 (USDA, 1954). These solutions were used to saturate the germination blotters and moisten the seeds during testing.
The protocol included random selection of 100 seeds per temperature per replicate per variety of seed; and saturating the blotter paper with distilled water or saline solution for each combination of salinity, replication, and variety within each temperature. Seeds were placed evenly across the test surface and each blotter was then rolled and folded in such a way as to keep the seeds enclosed. The rolled blotter papers were placed in plastic bags labeled with seed type, salinity level, test temperature, and replication number. Each bag was then placed in the appropriate temperature-controlled germination chamber.
Six observations were made during the 21-day period to evaluate seed germination. The criteria used to evaluate seed germination were a primary root at least 5 mm long in the root hair zone, elongating hypocotyls above the root hair zone, and no lesions or decay from mold.
|
Table 1. Mean percent germination for chile seed incubated in a grow the chamber at either 15°C (59°F ) or 20°C (68°F) and at five salinity levels over time. |
||||||||||||||
|
|
|
Temperature (°C/°F) |
||||||||||||
|
|
|
15/59 |
20/68 |
|||||||||||
|
|
|
Salinity Level (dS/m) |
||||||||||||
|
|
|
1 |
3 |
5 |
7 |
9 |
1 |
3 |
5 |
7 |
9 |
|||
|
Chile Type |
Day |
% Germination |
||||||||||||
|
AZ-20 |
6 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
22.5 |
16.5 |
13.0 |
2.5 |
0.0 |
|||
|
|
9 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
78.5 |
69.5 |
68.0 |
65.8 |
34.8 |
|||
|
|
12 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
85.8 |
79.8 |
76.3 |
80.0 |
75.5 |
|||
|
|
15 |
0.3 |
0.5 |
0.5 |
0.0 |
0.0 |
88.3 |
85.0 |
79.8 |
84.8 |
80.0 |
|||
|
|
18 |
8.3 |
8.5 |
4.3 |
4.3 |
0.0 |
90.3 |
85.0 |
80.5 |
86.0 |
81.3 |
|||
|
|
21 |
52.3 |
48.8 |
38.3 |
25.3 |
25.3 |
89.8 |
86.5 |
82.0 |
85.0 |
83.5 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Large Red Thick |
6 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
3.3 |
3.8 |
1.0 |
0.0 |
0.0 |
|||
|
|
9 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
58.8 |
58.3 |
55.5 |
54.0 |
41.8 |
|||
|
|
12 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
75.3 |
75.0 |
71.5 |
74.8 |
73.0 |
|||
|
|
15 |
2.0 |
1.8 |
1.3 |
2.3 |
0.3 |
80.3 |
78.8 |
76.3 |
78.5 |
77.3 |
|||
|
|
18 |
11.0 |
13.5 |
17.0 |
13.0 |
8.5 |
80.8 |
80.0 |
77.5 |
80.0 |
78.5 |
|||
|
|
21 |
46.3 |
42.8 |
50.0 |
47.8 |
50.3 |
80.0 |
81.3 |
79.0 |
80.0 |
83.5 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Sonora |
6 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
20.3 |
12.3 |
8.0 |
2.5 |
0.0 |
|||
|
|
9 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
84.0 |
68.5 |
80.8 |
73.3 |
46.3 |
|||
|
|
12 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
87.3 |
84.5 |
83.8 |
80.5 |
75.5 |
|||
|
|
15 |
2.8 |
7.8 |
2.5 |
1.3 |
0.0 |
87.5 |
87.5 |
85.5 |
81.3 |
77.3 |
|||
|
|
18 |
43.0 |
51.0 |
42.8 |
26.8 |
12.0 |
87.5 |
88.0 |
85.5 |
81.3 |
77.3 |
|||
|
|
21 |
84.3 |
84.5 |
82.5 |
73.0 |
67.0 |
89.3 |
86.0 |
83.8 |
80.5 |
70.3 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
TAM |
6 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
1.8 |
2.3 |
0.8 |
0.0 |
0.0 |
|||
|
|
9 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
80.3 |
83.8 |
67.8 |
51.3 |
26.3 |
|||
|
|
12 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
96.8 |
98.5 |
95.5 |
94.3 |
94.8 |
|||
|
|
15 |
0.8 |
1.5 |
0.3 |
1.0 |
2.0 |
97.3 |
99.0 |
96.3 |
95.5 |
95.8 |
|||
|
|
18 |
16.3 |
16.8 |
14.5 |
12.0 |
11.0 |
97.3 |
99.0 |
96.3 |
95.5 |
95.8 |
|||
|
|
21 |
68.8 |
67.0 |
59.3 |
57.0 |
42.0 |
98.0 |
98.0 |
95.8 |
96.8 |
92.5 |
|||
| Analysis of Variance |
| Source of Variation | Temperature = 15°C (59°F) | Temperature = 20°C (68°F) |
| Pr>F | Pr>F* | |
| Salinity | 0.0001 | 0.0001 |
| Variety | 0.0001 | 0.0001 |
| Salinity X Variety | 0.0006 | 0.0001 |
| Time | 0.0001 | 0.0001 |
|
Table 2. Mean percentage difference in germination between 20°C (68°F) and 15°C (59°F) over time for each salinity level. |
||||||||||
|
|
|
Salinity Level |
||||||||
|
Chile Type |
Day |
1 |
3 |
5 |
7 |
9 |
||||
|
|
|
% Germination Difference [20°C (68°F) – 15°C (59°F)] |
||||||||
|
AZ-20 |
6 |
22.5 |
16.5 |
13.0 |
2.5 |
0.0 |
||||
|
|
9 |
78.5 |
69.5 |
68.0 |
65.8 |
34.8 |
||||
|
|
12 |
85.8 |
79.8 |
76.3 |
80.0 |
75.5 |
||||
|
|
15 |
88.0 |
84.5 |
79.3 |
84.8 |
80.0 |
||||
|
|
18 |
82.0 |
76.5 |
76.3 |
81.8 |
81.3 |
||||
|
|
21 |
37.5 |
37.8 |
43.8 |
59.8 |
58.3 |
||||
|
|
|
|
|
|
|
|
||||
|
Large Red Thick |
6 |
3.3 |
3.8 |
1.0 |
0.0 |
0.0 |
||||
|
|
9 |
58.8 |
58.3 |
55.5 |
54.0 |
41.8 |
||||
|
|
12 |
75.3 |
75.0 |
71.5 |
74.8 |
73.0 |
||||
|
|
15 |
78.3 |
77.0 |
75.0 |
76.3 |
77.0 |
||||
|
|
18 |
69.8 |
66.5 |
60.5 |
67.0 |
70.0 |
||||
|
|
21 |
33.8 |
38.5 |
29.0 |
32.3 |
33.3 |
||||
|
|
|
|
|
|
|
|
||||
|
Sonora |
6 |
20.3 |
12.3 |
8.0 |
2.5 |
0.0 |
||||
|
|
9 |
84.0 |
68.5 |
80.8 |
73.3 |
46.3 |
||||
|
|
12 |
87.3 |
84.5 |
83.8 |
80.5 |
75.5 |
||||
|
|
15 |
84.8 |
79.8 |
83.0 |
80.0 |
77.3 |
||||
|
|
18 |
44.5 |
37.0 |
42.8 |
54.5 |
65.3 |
||||
|
|
21 |
5.0 |
1.5 |
1.3 |
7.5 |
3.3 |
||||
|
|
|
|
|
|
|
|
||||
|
TAM |
6 |
1.8 |
2.3 |
0.8 |
0.0 |
0.0 |
||||
|
|
9 |
80.3 |
83.8 |
67.8 |
51.3 |
26.3 |
||||
|
|
12 |
96.8 |
98.5 |
95.5 |
94.3 |
94.8 |
||||
|
|
15 |
96.5 |
97.5 |
96.0 |
94.5 |
93.8 |
||||
|
|
18 |
81.0 |
82.3 |
81.8 |
83.5 |
84.8 |
||||
|
|
21 |
29.3 |
31.0 |
36.5 |
39.8 |
50.5 |
||||
|
Table 3. Chile seed germination under standard testing procedures. |
||||||||||||
|
Day |
AZ-20 |
Large Red Thick |
Sonora |
TAM |
||||||||
|
|
]% Germination |
|||||||||||
|
6 |
63.3 |
12.0 |
41.5 |
35.5 |
||||||||
|
9 |
82.0 |
57.3 |
79.3 |
92.0 |
||||||||
|
12 |
87.5 |
71.8 |
80.3 |
96.5 |
||||||||
|
15 |
89.8 |
74.5 |
80.8 |
97.3 |
||||||||
|
18 |
90.0 |
76.3 |
81.3 |
97.3 |
||||||||
|
21 |
89.5 |
80.5 |
79.5 |
96.5 |
||||||||
|
|
|
|
|
|
||||||||
| Analysis of Variance | ||||||||||||
|
|
Pr>F |
|
|
|
||||||||
|
Chile Type |
0.0001 |
|
|
|
||||||||
|
Day |
0.0001 |
|
|
|
||||||||
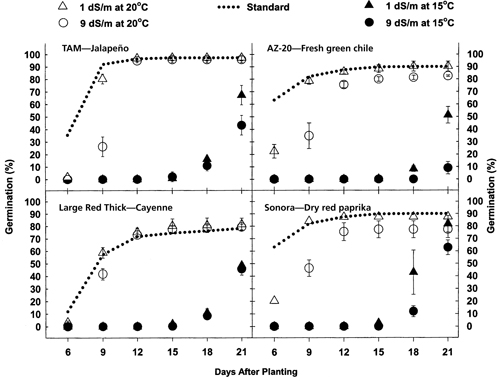
Figure 1. Chile seed germination for four chile types ('AZ-20', 'TAM', 'Sonora', and 'Large Thick Red') as affected by salinity and temperature over time. Standard protocol germination also is plotted as a reference to the temperature treatments,15°C (59°F) and 20°C (68°F).
Results
The results of this study indicated a highly significant (p=0.0001) relationship between salinity and chile type on germination (table 1). There were significant interactions between salinity and chile type and a significant effect of time on germination. There also were significant differences in germination between 10°C (50°F), 15oC (59oF), and 20oC (68oF) over time. There was no germination during the 21-day test for seed exposed to a constant 10oC (50°F) temperature treatment, regardless of salinity or variety. Germination levels at 15°C (59°F) were drastically lower than those at 20°C (68°F) during the first 18 days after placement, regardless of salinity or variety (fig. 1). After 21 days of incubation, there were some similarities in germination among salinity levels and varieties and some obvious differences (fig. 1). The results are summarized in table 2. ‘Sonora’ chile exhibited very little difference in total germination percentage across all salinity levels between 15°C (59°F) and 20°C (68°F) (<7.5% difference) (table 3). ‘AZ-20’ had the largest difference in germination among temperatures across all salinity levels (<59.8% difference).
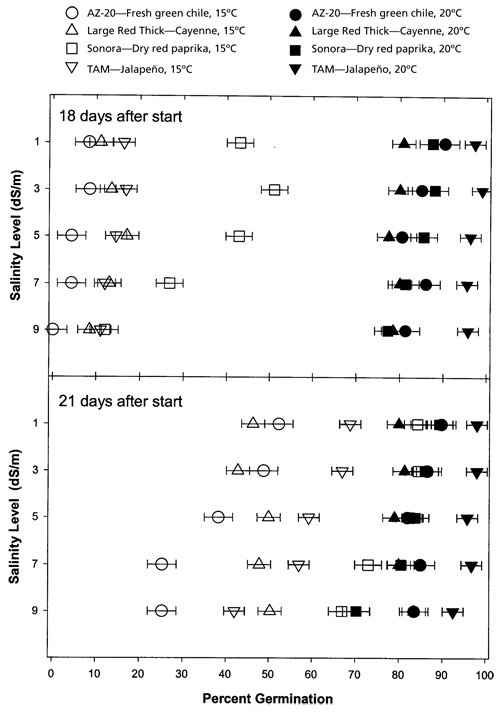
Figure 2. Chile seed germination 18 and 21 days after the start of the incubations at15°C (59°F) or 20°C (68°F) for 'AZ-20', 'Large Red Thick', 'Sonora', and 'TAM'.
For all tested chile types subjected to a constant 20°C (68°F), 90% of maximum germination occurred within 12 days compared to 0% in 12 days when incubated at 15°C (59°F) (figure 1). Fifteen days elapsed before seeds exposed to a constant 15°C (59°F) began to germinate. By day 21, seeds subjected to a constant 15°C (59°F) achieved only 30% of the germination level of seeds subjected to a constant 20oC (68°F), at salinity levels at or above 7 dS/m. Lower salinity levels at 15°C (59°F) permitted germination to reach a minimum of 58% of the maximum germination achieved at 20°C (68°F) (table 3). Only ‘Sonora’, the red chile variety, achieved a comparable 21-day germination percentage at both 15°C and 20°C (68°F) (fig. 1).
Germination at a constant 20°C (68°F) was affected by a saline solution of 9 dS/m during the first nine days of incubation. There was a small depression (<6.3%) in total germination due to the 9dS/m solution at 20°C (68°F) for all varieties except ‘Large Red Thick’. This cayenne chile type had 19% less germination in 9 dS/m water than in 1 dS/m water when incubated at 20°C (68°F). ‘AZ-20’, ‘Sonora’, and ‘TAM’ demonstrated reduced germination with increased salinity levels when incubated at 15°C (59°F). High levels of salinity at 15°C had no adverse effect on germination for the cayenne seed.
Germination also varied under standard protocols used for evaluating chile germination. After 21 days of incubation using distilled water, there were similar germination rates between ‘TAM’ and ‘AZ-20’ (93% average germination) and between ‘Sonora’ and ‘Large Red Thick’ (80% average germination) (table 3). The 13% difference in average germination between these two groups of chile was significant. Incubating the seed at 20°C (68°F) was similar to the standard test procedure (fig. 1). The largest difference between the constant 20°C (68°F) incubation and the standard procedure occurred during the first 9 days of incubation (fig. 1).
Discussion
Temperature had a major impact on seed germination. High levels of salinity affected germination throughout the incubation period. Therefore, it shouldn’t be a surprise that growers experience slow germination with early spring plantings when soil temperatures average 15°C. Prolonged contact with a soil environment without germination exposes seeds to diseases, insects, rodents, and other detrimental environmental factors. A best management practice would require an average soil temperature of 20°C (68°F) in the seed zone. Soil temperatures below this level most likely will slow seed germination and predispose seed to biological and environmental damage.
Salinity most likely will have less impact during germination under optimum soil temperature conditions. However, it can affect stand establishment during emergence. The effect of high salinity on seedlings in the cotyledon through true leaf stage is devastating. Furthermore, chile yield is reduced 14% for every unit of salinity above its threshold (1.7 dS/m) (Rhoades et al., 1992). Generally, pepper yield can be reduced 25% at soil salinity levels of 3 dS/m (Lorenz and Maynard, 1980). Field observations made during 1999–2001 confirm this response in New Mexico chile fields. Salinity damage often is mistaken for pathogens that cause damping off or other problems. Fields should be assessed for salinity and, if pathogens are suspected, a qualified plant pathologist should confirm their presence. An improvement to current guidelines could be realized with documenting salinity effects during chile stand establishment.
Under standard AOSA-approved conditions, the significant difference in germination between chile grown for fresh market (‘AZ-20’ and ‘TAM’) and the other two varieties suggests a difference in chile seed quality. How the seed is grown and prepared could account for the 13% difference in germination. This study would be improved by using seed lots and chile types that test similarly under standard germination procedures.
Future Research
Salinity continues to be a concern for chile producers. Evaluating emergence and establishment at different temperatures and soil salinity levels would improve in-field diagnosis of stand establishment problems. Field testing should be conducted to document yield depression due to salinity under real growing conditions.
Footnote
1This article was reviewed by Michael K. O'Neill, assistant professor, NMSU Agricultural Science Center at Farmington, N.M.; Denise McWilliams, Extension
agronomist, NMSU Extension Plant Sciences Department, Las Cruces, N.M.; Charles R. Glover, professor, NMSU Department of Agronomy and Horticulture,
Las Cruces, N.M.; and Joe N. Corgan, professor emeritus, NMSU Department of Agronomy and Horticulture, Las Cruces, N.M.
References
Bosland, P.W., A.L. Bailey, and J. Iglesias-Olivas. 1996 . Capsicum pepper varieties and classifications. Ext. Circ. 530. N.M. Coop. Ext. Ser., Las Cruces, N.M.
Bosland, P.W. and E.J. Voltava. 2000. Peppers: Vegetable and spice capsicums. CABI Publishing, London.
DeWitt, D. and P.W. Bosland. 1996. Peppers of the world. Ten Speed Press, Berkeley, Calif.
Lorenz, A.A. and D.N. Maynard. 1980. Knott's handbook for vegetable growers. John Wiley and Sons, Wiley Interscience Publication, N.Y.
Rhoades, J.D., A. Kandiah, and A.M. Mashali. 1992. The use of saline waters for crop production. Food and Agriculture Organization of the United Nations (FAO) Irrigation and Drainage Paper 48. FAO, Rome, Italy.
Tanksley, S.D., and G.C. Phillips. 1985. Evaluation of the potential for genetic improvement of salt tolerance in chile pepper (Capsium annuum) using wild germplasm and cell selection procedures. Report WRRI Proj. No. 1345646. N.M. Water Resour. Res. Instit., Las Cruces, N.M.
U.S. Department of Agriculture. 1954. Agricultural handbook No. 60, salinity laboratory handbook. U.S. Gov. Print. Office, Washington, D.C.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at aces.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
August 2002, Las Cruces, NM