Incidence of the Beet Leafhopper, Circulifer tenellus (Homoptera:Cicadellidae), in New Mexico Chile1,2
New Mexico Chile Task Force Report 12
Rebecca Creamer, Jared Carpenter and Jaime Rascon
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Assistant professor (creamer@taipan.nmsu.edu), research assistant (jarcarpe@nmsu.edu) and student aide, Department of Entomology, Plant Pathology and Weed Science, New Mexico State University, Las Cruces, N.M.
Introduction
Beet curly top virus (BCTV) epidemics have occurred sporadically in the southern New Mexico chile pepper growing areas since first reported in 1927 (Crawford, 1927). In three of the last 10 years, New Mexico chile sustained substantial losses due to BCTV. In 2001, a year with high BCTV incidence, the chile yield per acre was 12% lower than in 2000 or 2002, years with minimal BCTV pressure (NASS-USDA, 2003). Chile plants infected with BCTV often are stunted severely and have chlorotic leaves. If infected early, they do not produce fruit. When produced by plants infected later in the season, chile fruit is usually small and round. No effective control measures are known for BCTV in chile.
BCTV is a monopartite geminivirus of the genus Curtovirus, characterized by a circular ssDNA genome within twin spherical particles. Molecular characterization of BCTV in sugarbeet has demonstrated that the virus primarily exists as three strains (CFH, Worland and California/Logan) and variants of these strains (Stenger and McMahon, 1997). BCTV infects a broad range of hosts that includes crops and weeds in many plant families (Bennett, 1971).
BCTV is transmitted by the beet leafhopper, Circulifer tenellus (Baker), a member of the subfamily, Deltocephalinae, of the homopteran family, Cicadellidae. The beet leafhopper is endemic throughout the western and southwestern U.S., preferring arid and semiarid conditions. The leafhopper vector feeds and breeds on an extensive range of plant families (Cook, 1967). It exists as three morphological types: a summer morph, a winter morph and a migratory morph (Severin, 1921). The summer morphs survive 3-4 months, while the winter morphs live longer and consist primarily of mated overwintering females. Migratory morphs are capable of flying several hundred miles (Dorst and Davis, 1937).
Leafhopper flight patterns in California are well described: C. tenellus overwinters in the foothills on weeds where the nymphs presumably acquire virus from these hosts (Cook, 1967). As these weeds dry in the spring, mature leafhoppers migrate into the interior valleys, feeding on and infecting crops and weeds. Spring movements of leafhoppers in California also have been correlated with temperature accumulation (Cook, 1945). The leafhoppers progress through several generations before moving back into the foothills in the fall.
The beet leafhoppers’ migratory patterns in New Mexico are not as well studied. Romney (1939) reported that the Rio Grande Valley was a spring and summer beet leafhopper breeding area, where the insects undergo three to five breeding cycles. Leafhoppers were thought to spread in the spring from this area to areas north and east, including northern New Mexico, west Texas and portions of Oklahoma, Kansas and Colorado. However, New Mexico land use patterns have changed dramatically since the 1930s when sugarbeet was a primary crop.
The beet leafhoppers’ weed host range is well-documented (Bennett, 1971), although data on relative BCTV incidence in weeds is limited (Creamer et al., 1996). Romney (1939) reported that a perennial mustard was the most important overwintering host in much of the New Mexico breeding area in the 1930s. That weed is now scarce. Given the changes in crops and weeds in the past 60 years and the reoccurring losses to chile due to the virus, we felt a New Mexico beet leafhopper status update was necessary. This paper reports beet leafhoppers’ incidence in chile fields in two years, one with high curly top disease pressure and one with low pressure. In addition, we report the disease incidence in weed hosts for the two years.
Methods and materials
We assayed leafhoppers’ incidence in chile by trapping insects from chile field margins from February 2001 through December 2002. In 2001, we sampled 10 fields (five from each county). Since chile is not usually grown in the same field in consecutive years, we chose chile fields located near those sampled in 2001 for testing in 2002. We placed four yellow sticky traps(20 x 25 cm) approximately 61 cm from the ground at the margins of each test field. Traps were changed every two weeks and leafhoppers identified and counted. Meteorological data were gathered from weather stations near the two groups of chile fields. Degree days were calculated using the single sine method, using a 50°F (10°C) lower threshold.
We estimated BCTV incidence in chile fields by counting the symptomatic plants among 100 randomly chosen plants in the test fields and selected additional fields. This method underestimates the total amount of virus infection, since symptomatic plants are removed during crop thinning.
At two-week intervals, weeds were collected from the margins of each field. After the plants were identified to species, 0.5-g leaf samples were ground in liquid nitrogen and total DNA extracted (Palmer et al., 1998). BCTV was amplified by polymerase chain reaction (PCR) using a viral specific primer set 5’-GTGGATCAATTTCCAGACAATTATC-3’ and 5’-CCCATAAGAGCCATATCAAACTTC-3’, which amplifies a portion of the coat protein gene. Primers that specifically amplify the Worland strain (5’-CCAGGACTTAAGGGCTTCATTT-3’ and 5’-GGAGGCCAGCAGACGGCTAA-3’) and CFH strain (5’-CTACGTCATCAATGACGTT-3’ and 5’-AGCTCCTCGCTATAAATACA-3’) were used to determine the BCTV strain after plants had been determined to contain the virus. PCR reactions were carried out in a total volume of 50 µl containing 20.5 µl H2O, 5 µl 10X buffer (100 mM Tris-HCl, pH 8.3 500 mM KCl, 0.01% gelatin), 1 µl 10 mM dNTPs, 3 µl 25 mM MgCl2, 5 µl each of 5mM primers, 4 U Taq DNA polymerase and 10 µl of 1:10 dilution of purified DNA.
Amplification using primers to detect all BCTV strains and BCTV-Worland was carried out with the following parameters: 35 cycles consisting of 94°C for 30 sec, 59°C for 60 sec and 72°C for 90 sec. A final extension of 72°C for 5 min followed. Amplification to detect BCTV-CFH was done similarly, except that the annealing step was done at 53°C for 60 sec. Amplification products were separated by electrophoresis on a 2% agarose gel, and the bands were visualized with ethidium bromide.
Results and discussions
The beet leafhopper vector of BCTV was trapped from all fields assessed during the nearly two-year period. C. tenellus appeared in chile fields in Doña Ana County during the end of March and beginning of April in 2001 and 2002, and in Luna County during early-to-middle May in 2001 and 2002 (figs. 1 and 2). Leafhopper numbers in 2002 peaked in June in Doña Ana County, while in Luna County, they peaked in August. In both areas surveyed, the number of leafhoppers trapped per field decreased below 20 by the end of October 2002, while in 2001, leafhopper numbers did not drop below 20 per field until mid-December. In both years, more leafhoppers were trapped from Doña Ana County fields than from Luna County fields.
The maximum number of leafhoppers trapped in a field in 2002 (3,500/field, fig. 3) was substantially more than in 2001 (145/field). In 2001, many more leafhoppers were trapped in one Rincon, N.M., field than in any other field from mid-April through July (fig. 3), although disease incidence was not different from the other Doña Ana County fields tested.
Although increased numbers of beet leafhoppers appeared in Doña Ana County chile fields at least a month earlier than Luna County fields, the 2001 and 2002 January-to-April temperatures (accumulated degree days) were similar between the two areas. Doña Ana County fields accumulated 411°F (210°C) degree days (DD) from January 1 through March 31, 2001, and 398°F (203°C) DD for the interval during 2002, compared to 388°F (196°C) DD for Luna County for the same three months in 2001 and 442°F (226°C) DD in 2002. This suggests that temperature was not the primary factor in determining beet leafhopper flight in this study. This differs from Cook’s (1945) results, which suggest that beet leafhoppers leave their breeding grounds as soon as they achieve maturity.
During the January-to-April period for both years, Luna CounChenopodiumty received more precipitation (1.36 in., Jan. 1 – March 31, 2001; 1.35 in., Jan. 1 –March 1, 2002) than Doña Ana County during the same interval (0.99 in., 2001; 0.93 in., 2002). The extra precipitation could have delayed the leafhoppers’ departure from overwintering weed hosts in Luna County compared to Doña Ana County, by delaying the drying of the weeds. Drying of the overwintering weed hosts is thought to be a primary factor contributing to spring flights (Carter, 1930; Severin, 1933).
BCTV incidence in chile in 2002 (0.5-1%) was substantially less than in 2001 (30-50%). The BCTV incidence in weeds was correspondingly lower. The incidence in weeds is likely a more accurate estimate of the actual level of disease in the environment since infected weeds do not show disease symptoms, thus sampling is unbiased. We found 1.3% incidence of BCTV in weeds in 2001 (9 infected / 686 plants tested) compared to 0.06% in 2002 (1 infected / 1,768 plants tested).
Four weed species were found to be infected with BCTV (table 1). These have been reported previously as hosts for the virus (Bennett, 1971). All have been reported as infected in field collections in California (Creamer et al., 1996). Other frequently tested plants that did not test positive for BCTV infection included bindweed (Convolulus arvensis L.) (119 plants tested); spurred anoda (Anoda cristata (L.) Schlecht) (114 plants tested); ground cherry (Physalis wrightii Gray) (197 plants tested); and Russian thistle (Salsola iberica Senne & Pau) (315 plants tested). The lack of infected Russian thistle in the field, even though collected for 10 months of the year, is similar to findings in California, where although the plant was frequently collected, it was never found to be infected with BCTV (Creamer et al., 1996).
All the BCTV-infected plants were found to contain the Worland strain of the virus. In addition, one Chenopodium sp. was found to be infected with the CFH strain. Infected plants were collected in June, July, August and September, 2001, and August, 2002. The 2002 virus-infected sample was collected from Doña Ana County. In 2001, eight infected samples were collected from Doña Ana County and one from Luna County.
The collection of BCTV-infected weeds at the margins of chile fields through much of the 2001 growing season emphasizes the need for more stringent weed control. The four species of infected weeds are all reported to be beet leafhopper and BCTV hosts (Cook, 1967) and, thus, could serve as a virus source for infection into chile fields.
|
Table 1. BCTV-infected weeds collected in 2001-2002.
|
||
|
Species
|
Number infected 2001/ | Number infected 2002 |
|
Amaranthus sp. (pigweed)
|
2/237 | 0/174 |
|
Sisymbrium irio L. (London rocket)
|
2/115 | 0/252 |
|
Chenopodium sp. (lambsquarters)
|
2/57 | 0/125 |
|
Kochia scoparia (L.) (Schrader)
|
3/18 | 1/195 |
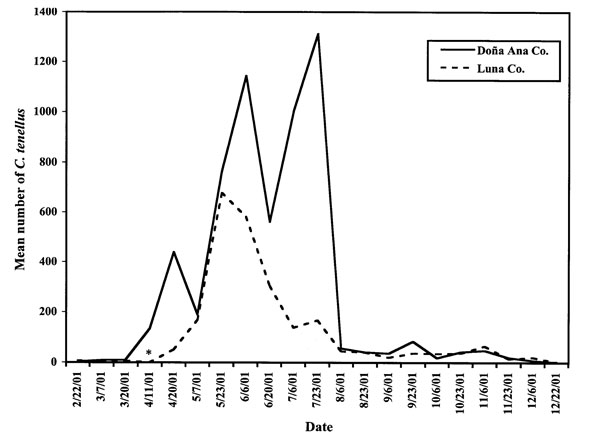
Figure 1. Mean numbers of adult Circulifer tenellus caught on yellow sticky traps from the margins of chile fields in two New Mexico counties in 2001. Note maximum value on Y axis. Asterisk denotes date when no data were available for Luna County due to severe dust storms.
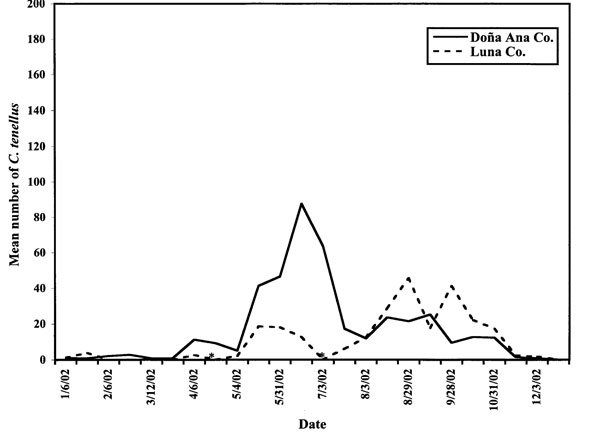
Figure 2. Mean numbers of adult Circulifer tenellus caught on yellow sticky traps from the margins of chile fields in two New Mexico counties in 2002. Asterisks denote dates when no data were available for Luna County due to severe dust storms.
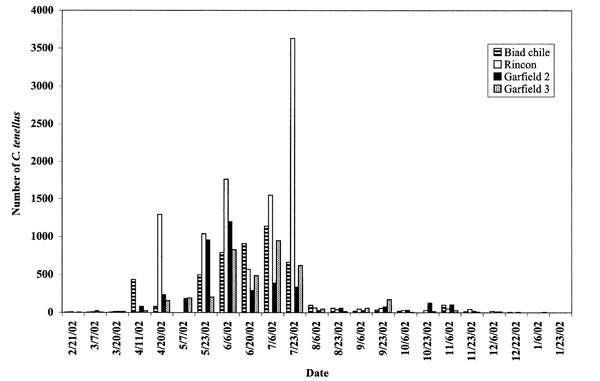
Figure 3. Numbers of adult Circulifer tenellus caught on yellow sticky traps from the margins of four chile fields in Doña Ana County in 2001. Note maximum value on Y axis.
Footnotes
1 This study was published in Southwestern Entomologist, Sept. 2003, 28(3), 177-182. (back to top)
2The authors thank Marvin Clary, Vince Hernandez and the New Mexico Chile Task Force for their help in selecting and acquiring test fields; Dayna Drollinger, Attelia Lewis and D. P. Garrido for help with extracting nucleic acid and carrying out polymerase chain reaction (PCR); and the New Mexico Agricultural Experiment Station for funding this work. (back to top)
Literature cited
Bennett, C. W. 1971. The curly top disease of sugarbeet and other plants. The Amer. Phytopathol. Soc. Monogr. No. 7.
Carter, W. 1930. Ecological studies of the beet leafhopper. U.S.D.A. Tech. Bull. 206. 115 pp.
Cook W. C. 1945. The relation of spring movements of the beet leafhopper (Eutettix tenellus Baker) in central California to temperature accumulations (Homoptera). Ann. Entomol. Soc. Amer. 38: 149-162.
Cook, W. C. 1967. Life history, host plants, and migrations of the beet leafhopper in the western United States. U.S.D.A. Tech. Bull. 1365. 122 pp.
Crawford, R. F. 1927. Curly top in New Mexico. U.S.D.A. Off. Rec. 6: 8.
Creamer, R., Luque-Williams, M., and Howo, M. 1996. Epidemiology and incidence of beet curly top geminivirus in naturally infected weed hosts. Plant Dis. 80: 533-535.
Dorst, H.E., and Davis, E. W. 1937. Tracing long-distance movements of beet leafhopper in the desert. J. Econ. Entomol. 30: 948-954.
National Agricultural Statistics Service. 2003. United States Department of Agriculture, National Vegetable Estimation Program.
Palmer, K. E., Schnippenkoetter, W. H., and Rybicki, E. P. 1998. Geminivirus isolation and DNA extraction. pp. 41-52. In G. D. Foster and S. C. Taylor (eds.) Methods in Molecular Biology, Vol. 81: Plant Virology Protocols: From Virus Isolation to Transgenic Resistance. Humana Press Inc., Totowa, NJ.
Romney, V. E. 1939. Breeding areas and economic distribution of the beet leafhopper in New Mexico, southern Colorado, and western Texas. U.S.D.A. Cir. 518. 14 pp.
Severin, H. H. P. 1921. Summary of the life history of the beet leafhopper (Eutettix tenella Baker). J. Econ. Entomol. 14: 433-436.
Severin, H. H. P. 1933. Field observations of the beet leafhopper, Eutettix tenellus, in California. Hilgardia 7: 281-360.
Stenger, D. C., and McMahon, C. L. 1997. Genotypic diversity of beet curly top virus populations in the western United States. Phytopathology 87: 737-744.
For more on this topic, see the following publications:
NMCA-30: Bacterial Leaf Spot of Chile Pepper: A Short Guide for Growers
https://pubs.nmsu.edu/research/horticulture/NMCA30/index.html
NMCA-33: Evaluations of Chloropicrin Fumigants for Management of Soil-Borne Pathogens in Chile (Capsicum annuum L.)
https://pubs.nmsu.edu/research/horticulture/NMCA33/index.html
CR-549:The Chile Pepper Diseases
https://pubs.nmsu.edu/_circulars/circ549.html
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at aces.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
July 2004


