Guide H-187
Kristen Bowers and Joanie King
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Respectively, Research Scientist, Entomology, Plant Pathology and Weed Science; Extension Entomologist and Assistant Professor, Extension Plant Sciences and Entomology, Plant Pathology and Weed Science. New Mexico State University. (Print-Friendly PDF)

Figure 1. Puncturevine plants growing at the edge of a parking lot; note yellow flowers.
Introduction
Puncturevine is a summer annual plant native to Asia, southern Europe, and northern Africa. It particularly thrives in hot, dry weather, and is commonly found in cultivated and otherwise disturbed areas (e.g., along roadsides and abandoned fields) (Figure 1). Puncturevine spreads along bare ground in mats that can reach five or more feet in diameter. The stems and leaves are covered with hairs, and its flowers are bright yellow (Figure 2a and 2b). Germination of puncturevine begins in early spring as temperatures warm and soil moisture is present. In southern New Mexico, germination can begin as early as March. These plants can produce seeds within two to three weeks of germination. The seed pods, known as goatheads, are covered with hard spines that injure livestock, lower forage quality, and puncture bicycle tires (Figures 3, 4a, and 4b). The spiny goathead has five segments, each of which contains between one to five individual seeds. When these seeds mature, they fall off the plant and the individual seed segments separate. The seeds can survive in the soil for at least eight years, producing a large supply of seeds to reinfest an area. However, the plant is not cold hardy and will die back with frost.

Figure 2a and 2b. Puncturevine plants with seed pods (goatheads).

Figure 3. Immature goathead
Puncturevine was an accidental introduction to the United States, possibly on the wool of sheep imported from Mediterranean Europe. This weed was recognized as problematic as early as 1912 in California, although ranchers reported sightings of it decades prior. Currently, puncturevine can be found in all of the U.S. states except Alaska, West Virginia, and Georgia and it has become a much bigger nuisance west of the Mississippi River. Despite the use of chemical and cultural weed control methods, puncturevine continued to spread across the United States in the early 20th century. Puncturevine became such a widespread weed because of its ability to grow in dry and disturbed soils, the large number of seeds it produces, and the ability of the seeds to attach themselves to people, animals, and vehicle tires—thus spreading to new locations (Figure 4a and 4b).
As a method of spread control, scientists have searched for biological control agents (i.e. host specific insects) that can feed on and kill puncturevine seed plants. In the 1950s, puncturevine plants across Europe and Asia were examined for damaging insects; from these surveys, two weevils were chosen as biological control agents: a seed feeding weevil (Microlarinus lareynii) and a stem mining weevil (Microlarinus lypriformis), both of the Coleoptera order and Curculionidae family (Figures 5a and 5b).

Figure 4a. Car tire covered in goatheads (photo by Ethelbert Johnson). 4b. Bottom of a shoe covered in goatheads.
Distribution
Both species of Microlarinus weevils were originally imported from Italy and released in California during the summer of 1961. Subsequent releases occurred across many western states in the early 1960s, including Colorado, Idaho, Kansas, Montana, Nebraska, New Mexico, Oklahoma, Oregon, Texas, Utah, Washington, Wyoming, and Hawaii. Follow-up surveys indicated that weevils had established and spread to adjacent counties in many of those locations.
A survey of puncturevine infestations across New Mexico and Colorado in 2022 found weevil damage and live weevils in nearly all survey locations. At every location with weevils, seed weevils were more common than stem weevils. In 2023, surveys of puncturevine in Idaho, Oregon, Montana, and Wyoming found no weevils or weevil damage in these locations. Researchers are currently investigating whether cold hardiness or other factors have prevented weevil establishment north of Colorado.
Description
Puncturevine Weevil Description
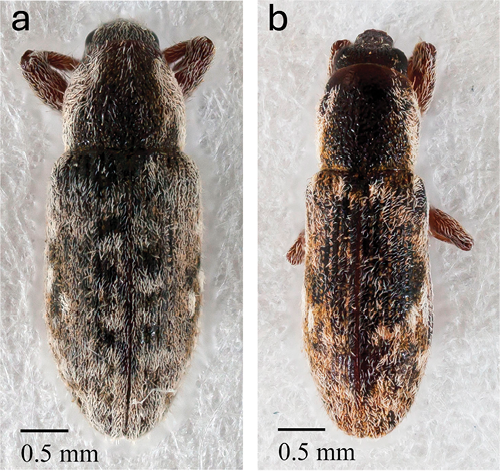
Figure 5a. Adult Microlarinus lareynii and 5b. Microlarinus lypriformis (photo by Joel DuBois).
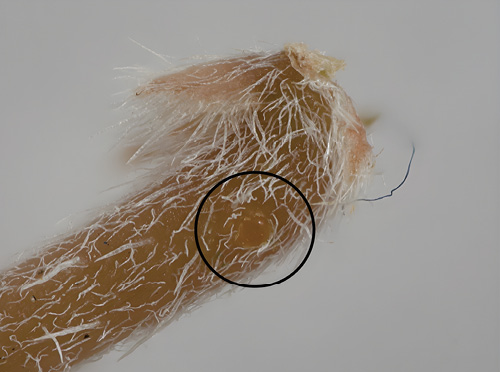
Figure 6. Stem weevil egg (photo by Helen Vessels and Esther Heerema).
Eggs of both weevils are cream to pale yellow colored, oval shaped, and about 0.55 mm or 1/50 of an inch long (Figures 6 and 7).
Larvae are white to cream colored with a light brown head capsule. The larvae are “C” shaped, wider at the head, and narrower towards the abdomen (Figures 8 and 9).
Pupae are about 4.7 mm or 1/5 inch long and white or light yellow.
Puncturevine seed weevil adults are 6.35 mm or ¼ inch long or less, and droplet shaped, with abdomens that are wider than their heads. Adult weevils are brown and covered with white hairs which may give them a “shaggy” appearance (Figures 5a and 10).
Puncturevine stem weevil adults are less than ¼ inch long, and cylindrically shaped, with their bodies being a uniform width. Adult stem weevils are also brown and covered in white hairs but may appear less “shaggy” than seed weevils (Figures 5b and 10).
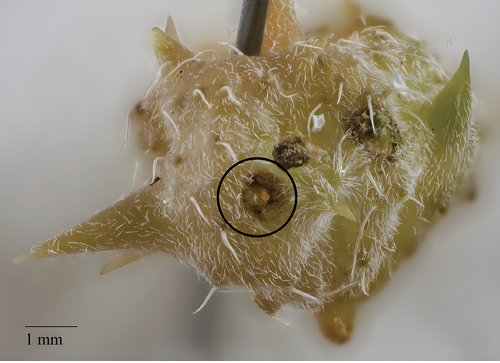
Figure 7. Seed weevil egg (photo by Helen Vessels and Esther Heerema)
Life Cycle
The common names of the weevils (puncturevine seed weevil and puncturevine stem weevil) (Figure 10) refer to the plant part that is consumed by the immature weevils. Although the immature weevils feed on different parts of their host plants, they have similar lifecycles.

Figure 8. Seed weevil larva (photo by Joel DuBois)

Figure 9. Stem weevil larva (photo by Joel DuBois)
Puncturevine Seed Weevil (Microlarinus lareynii) Lifecycle
A female weevil chews a pit in a young, tender goathead, which contains the plant’s seeds, and lays a single egg in each pit; she then covers the egg with fecal matter. The eggs hatch in 2 to 3 days and then the larvae feed on the seeds inside the pod (Figure 8). Multiple larvae can develop within a single goathead. The immature weevils complete their development inside the seed. Seed weevils can develop from egg to adult in 25 to 30 days, with weevils developing more quickly in warmer conditions. Once matured, weevils will chew an exit hole in the goathead and emerge. Adult weevils feed on puncturevine stems and foliage. New adult weevils will mate within a few days and female weevils will begin to lay eggs within approximately one week. The average female can lay about 200 eggs, with most of them laid within the first month of adulthood; eventually, egg laying declines as the female weevil ages. Weevils can live an average of 96 days, with a maximum lifespan of over 240 days. In warmer regions, weevils can have up to four generations in their lifespan, while in more temperate climates, they typically have only one generation.
Puncturevine Stem Weevil (Microlarinus lypriformis) Lifecycle
A female stem weevil chews a pit in the underside of the puncturevine stem and lays a single egg in each pit; she then covers the egg with fecal matter (Figure 11). The egg will hatch after 2-3 days, and the immature weevil will develop within the stem (Figure 9). Stem weevil development from egg to adult can take 45 to 51 days, or about two weeks longer than seed weevils. Once matured, weevils chew an exit hole in the stem and feed on leaflets and stems. As with the seed weevils, the stem weevils will mate and begin laying eggs within a week of adult emergence with the correct plant and environmental cues.

Figure 10. Adult seed weevil (left) and stem weevil (right) (photo by Helen Vessels).

Figure 11. Stem weevil egg covered with fecal pat.
Hosts
The host of puncturevine weevils is puncturevine. In the absence of puncturevine, weevils will attempt to feed on plants other than puncturevine. In forced feeding tests, seed weevils laid eggs on two species of plants in addition to puncturevine: Kallstroemia grandiflora and K. californica, which are both plants closely related to puncturevine. Kallstroemia paviflora was not tested. During these tests, seed weevil larvae were only able to complete development on K. grandiflora. The stem weevils laid eggs on puncturevine and on Zygophyllum faboga, but larvae died before completing development on Z. faboga. Neither species of weevil laid eggs on any of the other 39 plant species tested, including creosote bush, Larrea tridentata. The failure of weevils to oviposit on these plants suggest that these plants are not hosts for the weevils.

Figure 12. Puncturevine seedling.
Impact of puncturevine weevils
Several studies have assessed the impact of puncturevine weevils on the reproduction and survival of puncturevine plants. Weevils contributed to the death of about one third to one half of puncturevine plants in research plots by late August, even when plants received supplemental irrigation. Overall, the combined effect of stem and seed weevils reduced flower production by 29% in irrigated plots and 60% in unirrigated plots.
In some locations, up to 80% of goatheads exhibit symptoms of seed weevil damage. Additionally, goatheads affected by seed weevil feeding experience reduced seed production and lower germination rates, even for seeds that appear undamaged. Stem weevils reduced plant biomass and plant survival, especially under dry (unirrigated) conditions.
Anecdotal reports of puncturevine weevil impact document reduced use of herbicides to control puncturevine in locations with abundant weevils. The personnel costs of surveying and testing weevils prior to release were approximately $280,000, which equates to 3.5 scientists at $80,000 per year, in 1976 dollars.

Figure 13. Feeding scars from adult weevils.

Figure 14. Stem weevil exit hole (circled).
Management of puncturevine and puncturevine weevils
Mechanical Control
Mature puncturevine seeds are dormant throughout the winter. Seedlings start to grow in the early spring in locations that receive rain or irrigation water (Figure 12). Seedlings are easy to pull by hand and should be removed as soon as they appear because plants can produce seeds very quickly (within 2 to 3 weeks).
Seed weevils do not attack mature seeds, so collecting mature seeds in the fall and winter will reduce the number of seeds that germinate the following year. It is easiest to collect seeds after frost has killed the plants and the dry seeds can be vacuumed up with a shop vac or collected from the ground by pressing pieces of Styrofoam or old carpeting on the ground which will pick up the mature seeds.
Scouting for Weevils
Adult weevils will be able to feed on small seedlings but won’t lay eggs until stems and seeds are big enough to support the development of immature weevils. Adult puncturevine weevils are small and difficult to see, but damage from feeding and weevil development is distinctive. In the early spring, examine the undersides of stems for white feeding scars and exit holes from stem weevils (Figures 13 and 14). As goatheads develop, carefully break open the seeds and examine for signs of internal feeding and exit holes (Figure 13). Seed weevils are much more abundant than stem weevils. Both species of weevils are active during the daytime.
Weevil Management
Adult weevils can be moved by gently digging up infested plants and placing the whole plant or plants in a paper bag. For example, a large paper yard trash bag or cardboard box works well for this. Weevils can be held in paper box or bag in a cool location, away from direct sunlight for at least a week. As the puncturevines dry out, adult weevils will tend to crawl up or towards light and can be collected by opening the box or bag and brushed or picked out and placed in patches of puncturevine. Adult weevils that emerge later in the summer or early fall will feed on plants but will not lay eggs until the next spring.
For further reading: https://plantclinic.nmsu.edu/documents/puncturevine-w-13.pdf
References
- Andres, L.A. (1976). Biological Control of Puncturevine, Tribulus terrestris (Zygophyllaceae): Post Introduction Collection Records of Microlarinus Spp. (Coleoptera: Curculionidae). In T.E. Freedman (Ed.), Proceedings of the IV International Symposium for the Biological Control of Weeds (pp. 132-136). https://bugwoodcloud.org/ibiocontrol/proceedings/pdf/4_132-136.pdf
- Andres, L.A. & Angalet, G.W. (1963). Notes on the Ecology and Host Specificity of Microlarinus lareynii and M. lypriformis (Coleoptera: Curculionidae) and the Biological Control of Puncture Vine, Tribulus Terrestris. Journal of Economic Entomology, 56, 333–340. https://doi.org/10.1093/jee/56.3.333
- Georgia, A. (1914). A Manual of Weeds With Descriptions of All the Most Pernicious and Troublesome Plants in the United States and Canada. MacMillian Company, New York. https://doi.org/10.5962/bhl.title.40611
- Huffaker, C., Ricker, D., & Kennett, C. (1961). Biological Control of Puncture Vine: With Imported Weevils. California Agriculture, 15, 11–12. https://californiaagriculture.org/article/113282-biological-control-of-puncture-vine-with-imported-weevils
- Huffaker, C., Hamai, J., & Nowierski, R.M. (1983). Biological Control of Puncturevine, Tribulus Terrestris in California After Twenty Years of Activity of Introduced Weevils. Entomophaga, 28, 387-400. https://doi.org/10.1007/bf02372193
- Johnson, E. (1932). The Puncture Vine in California. University of California Bulletin, 528. https://doi.org/10.5962/bhl.title.59300
- Kirkland, R.L., & Goeden, R.D. (1978a). Biology of Microlarinus lareynii (Col.: Curculionidae) on puncturevine in Southern California. Annals of the Entomological Society of America, 71, 13–18. https://doi.org/10.1093/aesa/71.1.13
- Kirkland, R.L., & Goeden, R.D. (1978b). Biology of Microlarinus Lypriformis (Col.: Curculionidae) on Puncturevine in Southern California. Annals of the Entomological Society of America, 71, 65–69. https://doi.org/10.1093/aesa/71.1.65
- Kirkland, R.L., & Goeden, R.D. (1978c). An Insecticidal-Check Study of the Biological Control of Puncturevine (Tribulus Terrestris) by Imported Weevils, Microlarinus Lareynii and M. Lypriformis (Col.: Curculionidae). Environmental Entomology, 7, 349- 354. https://doi.org/10.1093/ee/7.3.349
- Kirkland, R.L., & Goeden, R.D. (1977). Descriptions of the Immature Stages of Imported Puncturevine Weevils, Microlarinus Lareynii and M. Lypriformis. Annals of the Entomological Society of America, 70, 583–587. https://doi.org/10.1093/aesa/70.4.583
- Maddox, D.M. (1976). History of Weevils on Puncturevine in and Near the United States. Weed Science, 24, 414–419. https://doi.org/10.1017/s0043174500066297
- Maddox, D.M. (1980). Seed and Stem Weevils of Puncturevine: A Comparative Study of Impact, Interaction, and Insect Strategy. In E.S. Delfosse (Ed.), Proceedings of the V International Symposium for the Biological Control of Weeds, Brisbane Australia (pp. 447-467). https://bugwoodcloud.org/ibiocontrol/proceedings/pdf/5_447-467.pdf
- SEINet Portal Network. (n.d.). https://swbiodiversity.org/seinet/. Accessed on October 20, 2023.
- Villegas, B., Gibbs, C., Wilson, R., & Smith, N. (2008). Puncturevine Biological Workshop in Lassen County. In D.M. Woods (Ed.), Biological Control Program Annual Report 2007 (pp. 32-33). https://bugwoodcloud.org/resource/files/25317.pdf
- Winston, R.L., Schwarzländer, M., Hinz, H., Day, M., Cock, M.J., & Julien, M. (2014). Biological Control of Weeds: A World Catalogue of Agents and their Target Weeds. Forest Health Technology Enterprise Team, USDA Forest Service https://www.ibiocontrol.org/catalog/JulienCatalogueFHTET_2014_04.pdf

Joanie King is a Research Scientist in the Department of Entomology Plant Pathology and Weed Science at New Mexico State University. She received her M.S. in Ecology and Ph.D. in Entomology from the University of Florida. Dr. Bowers is interested in the adoption, integration, and economic impact of biological control of weeds and insects in rangelands and orchards in New Mexico.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu/
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
February 2025, Las Cruces, NM


