Guide H-176
Alissa J. Freeman, Miranda L. Kersten, Amanda Skidmore, and Marisa Y. Thompson
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Former Senior Program Specialist; Program Manager, Agricultural Science Center at Los Lunas; Former Urban Small Farm IPM Specialist; Extension Urban Horticulture Specialist, Department of Extension Plant Sciences. (Print Friendly PDF)
Table of Contents
Integrated pest management in the home garden
Insects that suck plant fluids
Integrated Pest Management in the Home Garden
Plants in the southwestern climate must overcome stress from abiotic (non-living) factors, such as temperature extremes, minimal precipitation, and poor soil quality. These stressors can make plants more susceptible to infestations from insect pests and other biotic (living) factors. Damage caused by garden pests can reduce crop yield, increase vulnerability to other pests and diseases, and may reduce plant vigor or lead to plant death. However, most insects are harmless to plants and may even be considered beneficial by providing pollination services, preying on pest populations, and helping with decomposition and nutrient cycling. Applying integrated pest management (IPM) practices in the garden or small farm is an effective way to address pest populations that minimizes harm to beneficial insect populations. The goal of IPM is not to eradicate pests but to provide healthy conditions for plants and to minimize pest populations. By developing a management strategy for pests, homeowners can reduce pest problems, create more sustainable growing systems, and increase their garden’s productivity.
A successful IPM program includes a combination of different control strategies to minimize chemical intervention and reduce health, environmental, and economic risks. The first steps toward implementing IPM in the home garden or small farm include (1) prevention – preventing pest outbreaks through proper plant selection, site selection, planting, and care; (2) monitoring – inspecting the garden for pests or signs of pest activity and determining if damage is enough to require intervention; (3) identification – correctly identifying the host plant and pest in the garden or small farm; and (4) management – choosing the appropriate IPM strategies to tackle the pest problem, whether the tactic is cultural, mechanical/physical, biological, or chemical. For more information on the benefits and employment of IPM in the home garden or small farm, see NMSU Extension Circular 655, Integrated Pest Management (IPM) for Home Gardeners (https://pubs.nmsu.edu/_circulars/CR655). The following are examples of IPM strategies.
Cultural—Modifying the natural environment to create a less suitable environment for pests.Examples of cultural control strategies include:
- Select plant species/cultivars that are resistant or tolerant to common stressors like pests, drought, disease, or temperature extremes.
- Practice good sanitation by removing infested plant material and debris or fallen, rotting fruit from the garden.
- Prevent plant stress through appropriate site selection, light and wind exposure, and watering requirements.
- Rotate crops and adjust planting and harvesting dates with stressors in mind.
Examples of mechanical control strategies include:
- Hand removal and destruction of identified insect pests.
- Barriers, such as row covers and screens, can be effective at protecting plants from pests. For plant species that require pollination, be sure to remove covers during bloom time. Be sure to bury the edges of row covers to further prevent entry of pests. Plants should be monitored even when barriers are used to ensure that no pests have made it past the barrier. If pests are locked in with the plants, they will be protected from natural enemies and their populations can grow unchecked.
- Prune out any infested plant material.
- Dislodge pests and egg cases on plants using a stream of high-pressure water.
- Trapping can be used to monitor pest populations to better time chemical applications; however, traps have minimal impact on reducing populations.
There are three types of biological control: classical biological control, augmentation biological control, and conservation biological control. Classical biological control involves the importation of exotic biological control agents with the intent to establish colonies that can contribute to long-term pest control where a pest is affecting the area. Augmentation biological control is the introduction of natural enemies that naturally reduce pest populations. Conservation biological control is the manipulation or modification of a habitat to encourage existing or new natural enemies—for example, incorporating different floral resources that provide habitat and food for natural enemies. For more information on using floral resources to attract natural enemies, see NMSU Extension Guide H-169, Using Insectary Plants to Attract and Sustain Beneficial Insects for Biological Pest Control (https://pubs.nmsu.edu/_h/H169/).
Examples of biological control strategies include:
- Plant a diversity of floral resources (pollen and nectar) that are available from early spring to fall to provide food and nesting resources that are attractive to natural enemy populations.
- Provide overwintering and nesting habitat, such as dead plant material, fallen logs, leaf litter, and bare ground.
- Avoid or limit the use of chemicals in the garden, which can harm beneficial insects.
- If not naturally present or in large enough numbers, introduce commercially available natural enemies to help control pest populations.
Chemical—Using naturally derived or synthetic compounds to control and suppress pest populations. For more information and details on chemical applications in the garden or small farm, see “Pesticides: Safe and Effective Use in the Home and Landscape” (http://ipm.ucanr.edu/PMG/PESTNOTES/pn74126.html).
Ideally, chemical control should be used only when other methods have failed. When pest populations reach the economic threshold—the point at which damage is severe enough that a control treatment is required for economic return—a chemical application may be warranted. Gardeners should monitor pest populations following pesticide applications to evaluate treatment success. Keep in mind that pesticide applications may harm both pests and beneficial insects, which can lead to secondary pest outbreaks due to the loss of natural enemy populations. Broad-spectrum pesticides, such as carbaryl, will kill insects indiscriminately, but selective pesticides, such as Bt (Bacillus thuringiensis), may be used to lessen impacts on beneficial insects. When using chemicals, use products that minimize adverse effects on beneficial insects, and check for label warnings about effects on pollinators. Organic options can be used by both organic and conventional gardeners. Below are best management practices to follow if chemical controls are needed.
Best management practices for chemical control strategies include:
- Correctly identify the pest to make an informed decision on the best chemical control. Not all products work against all pests due to chemical structure or formulation.
- Follow the chemical label instructions completely and carefully. The label is the law.
- Only use chemical controls during the correct life cycle stage of the pest. This can be determined through careful monitoring and timing.
- Be aware of recommended wind speeds and temperatures before applying chemicals to reduce unintentional human and environmental health risks, such as chemical drift.
- Avoid spraying when flowers are blooming to minimize the effects on beneficial insects. Consider spraying in the early morning or evening to reduce pollinator exposure. Consider spot treating only where infestations of the pest are most damaging. Narrow-spectrum or selective products like Bt are less harmful to natural enemies.
- Pyrethroid insecticides are less effective in high temperatures (80°F or higher) and should be used only in the early season.
- Be sure that the pesticide is labelled for the plant being treated and be aware of when the crop can be harvested following treatment.
- Contact insecticides must be applied directly to the insect pest to be effective.
- Consult your local county Extension office (http://aces.nmsu.edu/county/) for further information on what products may be suitable for your particular situation.
The pesticide recommendations in this publication are provided only as a guide. The authors and New Mexico State University assume no liability resulting from their use. Please be aware that pesticide labels and registration can change at any time; by law, it is the applicator’s responsibility to use pesticides ONLY according to the directions on the current label. Use pesticides selectively and carefully and follow recommended procedures for the safe storage and disposal of surplus pesticides and containers.
Using This Guide
This guide is intended to help with identifying garden insect pests that may be encountered in the home garden or small farm by providing identification tips of the pest and the damage they cause, as well as suggested IPM practices. This guide is categorized by the feeding strategy of the insect pest.
Insects That Suck Plant Fluids
Aphids
(Figures 1A and 1B)
Host: Fruits, vegetables, and landscape plants. Host plant preference varies among species.
Biology and Life Cycle: Female aphids are able to reproduce without males. Wingless females give live birth during most of the summer rather than laying eggs. Aphids can complete several generations within a season because of their ability to reproduce quickly. This can cause host plants to become overcrowded, which leads to a decline in vigor and results in adult aphids with wings that are able to travel to new plants. Some aphids are specific to one plant, while others alternate between multiple plants throughout their life cycle. Winged aphids are poor fliers but can glide with the wind, which allows them to disperse for miles, often to find their alternate plant host. Male nymphs are produced in the fall, triggered by a shortening day length. Males mature and mate with females to produce offspring that give rise to egg-producing young. Eggs live through the winter on the host plant, and females hatch in the spring and begin reproducing. Some aphid species may overwinter on a different host plant species than that used for the summer. The life cycle of aphids varies among species, although the above description is the most common life cycle for many aphid species.
Metamorphosis: Incomplete
Overwintering Stage: Egg
ID Tips: Aphids range between 2–4 mm (1/16–1/8 inch) long in size, with soft pear-shaped bodies. Coloration varies among species; colors include green, yellow, black, red, and brown. Although many aphids are wingless, winged aphids typically have a darker coloration. All aphids have cornicles at the end of the abdomen.
Damaging Stage: Nymph and adult
Damage: Aphids tend to cluster and feed on new growth—leaves, developing stems, bark, and roots. This feeding may cause stunted plant growth, wilting, leaf yellowing, leaf curling, or foliage loss. They can also act as a vector for plant viruses, such as cucumber mosaic virus, lettuce mosaic virus, and turnip mosaic virus. Squash, cucumber, pumpkins, melons, tomatoes, spinach, lettuce, and beets are all susceptible to viral transmission. Signs of viruses include leaf mottling, leaf curling, and misshapen fruit. Viral infection may also result in plant death. After feeding, aphids secrete a sticky, glossy waste product called honeydew that is high in sugar concentration; honeydew attracts ants and yellowjackets and can lead to fungal problems. Often, plants can withstand aphid infestations and require monitoring to determine if and when control strategies should be implemented.

Figure 1A. Aphids clustered on the underside of a leaf. Swollen aphids are parasitized by wasps. (Photo by Miranda Kersten, NMSU.)
IPM Strategies:
Cultural: Keep plants well-watered and fertilized. Aphids target plants exhibiting stress. Remove weeds from the garden that can harbor aphid populations.
Mechanical: Dislodge aphids using a stream of high-pressure water. Repeat this process daily, if needed, until populations decline. This method also helps remove sticky honeydew from plants. Floating row covers can be installed at the time of planting to protect plants from aphid infestations. Gardeners should regularly check plants to ensure no aphids have made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Depending on the presence and abundance of natural enemies, aphid infestations may be managed without human interference. Encourage beneficial insects such as lady beetles (both adult and larval stage)—a single lady beetle can consume thousands of aphids. Both the larval stage of syrphid flies and lacewings will also feed on aphids. Parasitoid wasps from the families Braconidae and Aphelinidae use aphids as their hosts. Parasitized aphids—called mummies—appear gray and swollen. Beauveria bassiana is a commercially available pathogen that can be used for aphid control. Several species of natural enemies are commercially available for supplemental releases.
Chemical: Conventional chemical controls, such as permethrin, can reduce aphid populations; however, these pesticides are highly toxic to beneficial insects.
Organic options: Use organic insecticides such as neem oil. Insecticidal soaps and horticultural oils can be effective chemicals to reduce aphid populations below damaging levels. Insecticidal soaps and oils are contact insecticides and will only kill aphids that come into direct contact. Apply to both sides of leaves for best control.

Figure 1B. Cornicles (black protrusions) on the abdomens of aphids. (Photo by Miranda Kersten, NMSU.)
Bagrada bug
(Bagrada hilaris; Figures 2A and 2B)Host: Plants in the mustard family (Brassicaceae) are the most common host, including Chinese greens, arugula, and mustards, as well as various weeds, such as London rocket, wild mustards, and pepperweed. Canola, potatoes, corn, sorghum, cotton, and some legumes are also susceptible to bagrada bug damage.
Biology and Life Cycle: Barrel-shaped eggs are laid individually or in small clusters on the undersides of leaves, on stems, and in the soil, hatching in as little as four days. Eggs gradually change color from white to orange as they mature. Nymphs go through five instars before developing into adults. Different life stages may all exist at the same time, and there may be several generations in a season.
Metamorphosis: Incomplete
Overwintering Stage: Adult
ID Tips: Adult bagrada bugs resemble another pest, harlequin bugs (Murgantia histrionica), but they are a quarter to a third of the size, with a 5–7 mm (1/4 inch) shield-shaped body. Bagrada bug adults are black with orange and white markings, while harlequin bugs lack these white markings. Nymphs are bright orange and become darker as they go through instar stages. Wing pads and white spots on the abdomen develop on older nymphs.

Figure 2A. Bagrada bug adults. (Photo by Gevork Arakelian, LA County Dept. of Agriculture, Bugwood.org.)
Damaging Stage: Nymph and adult
Damage: Bagrada bugs feed on plant material and seeds, causing leaf spotting and wilting, stunted growth, and plant death. Feeding damage resembles a starburst-shaped lesion. Damage can be more severe on young plants that are not as able to withstand damage from feeding.
IPM Strategies:
Cultural: Keep plants healthy through proper fertilization and watering throughout the season. Sturdy, vigorous plants are more able to withstand feeding from bagrada bugs. Remove weeds to eliminate overwintering habitat.
Mechanical: Hand pick bagrada bugs off plants and place in soapy water. Use hand vacuums when there are heavy infestations. Pyramid traps baited with crushed sweet alyssum may help attract and trap bagrada bugs and are available commercially. Use floating row covers to exclude adult and nymph bagrada bugs and regularly monitor plants to make sure no bagrada bugs have made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Bagrada bugs do not have a specific natural enemy in the United States, but may be fed on by spiders and other predaceous insects. Bagrada bug eggs are susceptible to parasitoids, such as flies in the Tachinidae and Sarcophagidae families and wasps in the Scelionidae family. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Chemical controls are typically not effective for bagrada bug control due to their tendency to fly away when disturbed.
Organic options: Insecticidal soaps and horticultural oils can be used; however, bagrada bug populations are difficult to control using chemical applications.

Figure 2B. Bagrada bug nymph shortly after molting. (Photo by Jennifer Carr, University of Florida, Bugwood.org.)
Beet leafhopper
(Circulifer tenellus; Figures 3A and 3B)
Host: Host plants vary, but commonly include tomatoes, peppers, beans, and beets.
Biology and Life Cycle: Adults disperse from overwintering habitat—typically winter annual mustards—to find new host plants to lay their eggs on in the early spring. Nymphs develop two to three months later, allowing for multiple generations within a season.
Metamorphosis: Incomplete
Overwintering Stage: Adult
ID Tips: Beet leafhoppers have a 3 mm (1/8 inch) wedge-shaped body. Coloration ranges from pale green to brown. When disturbed, beet leafhoppers will jump or fly away.

Figure 3A. Adult beet leafhopper perched on a leaf. (Photo by Miranda Kersten, NMSU.)
Damaging Stage: Nymph and adult
Damage: Piercing and sucking feeding by adults and nymphs can result in reduced plant vigor. This damage is often not severe and may go unnoticed; however, adults may act as a vector for viruses such as curly top virus, which results in stunted plant growth, yellow leaves with purple veins, and upward curling of the leaf. It can cause fruits to be misshapen and ripen prematurely, leading to high losses of plant yield.

Figure 3B. Bean plant (Phaseolus vulgaris) showing signs of leaf yellowing and cupping from curly top virus. (Photo by Howard F. Schwartz, Colorado State University, Bugwood.org.)
IPM Strategies:
Cultural: Monitor for signs of curly top virus. Remove plants that are exhibiting symptoms. Eliminate overwintering habitat by removing winter weeds.
Mechanical: Use floating row covers made of lightweight garden fabric to keep beet leafhoppers off plants during the growing season. Row covers may need to be used from the time of planting until the time of harvest. Be sure to monitor under the row covers to ensure that pests have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Natural enemies of beet leafhoppers include big-headed flies, ants, assassin bugs, and egg parasitoids (families Mymaridae and Trichogrammatidae). Beauveria bassiana may be applied to control beet leafhoppers. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Due to the wide range of hosts and dispersal modes of beet leafhoppers, chemicals are not recommended as an effective management strategy.
Squash bug
(Anasa tristis; Figures 4A and 4B)
Host: All species in the Cucurbitaceae family, such as squash, pumpkin, and melons.
Biology and Life Cycle: When adults emerge from their overwintering habitat, they locate host plants. Females begin laying eggs in June and continue through summer. Eggs are laid in small clusters of about 20 between the veins on the undersides of leaves and stems, where they form a nickel-sized cluster. After approximately ten days, eggs hatch and nymphs emerge, maturing in four to six weeks, with up to two to three generations in a season depending on climatic conditions. Adults overwinter in sheltered habitat, such as rocks, buildings, and plant debris. Nymphs are unable to survive freezing temperatures.

Figure 4A. Squash bug ovipositing eggs on the underside of a leaf in typical V-formation following leaf venation. (Photo by Gerald Holmes, California Polytechnic State University at San Luis Obispo, Bugwood.org.)
Metamorphosis: Incomplete
Overwintering Stage: Adult
ID Tips: Adults have a 16 mm (5/8 inch) long body with a flat back. Coloration ranges from gray to black, with alternating orange and brown bands along the edge and underside of body. Oval-shaped eggs are 1.5 mm (1/16 inch) in size, yellow to bronze colored, and typically clustered along the undersides of leaf veins in nickel-sized clusters.
Damaging Stage: Nymph and adult
Damage: Squash bugs suck nutrients from leaves, which results in yellow or brown spots on the leaves and plant wilting. Younger plants and developing fruit are more susceptible to damage from squash bugs. Squash bugs also vector Serratia marcescens, a bacterium that causes cucurbit yellow vine disease.

Figure 4B. Squash bug infestation on a pumpkin. (Photo by Amanda Skidmore, NMSU.)
IPM Strategies:
Cultural: Keep plants healthy through proper fertilization and watering throughout the season. Sturdy, vigorous plants are able to withstand squash bug damage.
Mechanical: Monitor leaves for squash bug eggs throughout the season. Crush eggs on the undersides of stems and leaves. Nymphs and adults disperse when disturbed, making them more difficult to control. Nymphs are easier to kill than adults, so early detection is key. Boards and newspapers can be used as traps. Squash bugs will hide under these materials at night, allowing the possibility of removal in the morning. Place adults and nymphs in soapy water or crush to kill. Row covers can be used when the plant is not flowering and should be regularly monitored to make sure squash bugs have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Tachinid fly adults will lay eggs on the nymph and adult stages of squash bugs, and the hatched larvae will later consume the squash bug host. Other predators include spiders, ground beetles, and robber flies. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Pesticides should only be applied early in the season due to reduced effectiveness in higher temperatures. Apply pesticides early in the morning or late in the evening when squash bugs are inactive. Recommended pesticides include carbaryl, permethrin, bifenthrin, and esfenvalerate; however, these pesticides are highly toxic to beneficial insects.
Organic options: Apply diatomaceous earth (DE) around the base of your plants. Squash bugs that come into contact with DE will become dehydrated and will desiccate.
Thrips
(Figures 5A and 5B)
Host: Beans, carrots, squash, tomatoes, and many other garden vegetables and flowers.
Biology and Life Cycle: Eggs are laid in plant tissue and emerge two to four days later. After the second instar (two days after emergence), late-instar larvae go through two non-feeding stages (prepupal and pupal) before developing into adults. Several generations exist within a season.
Metamorphosis: Incomplete
Overwintering Stage: Adult
ID Tips: Adult thrips have long, slender bodies that are typically less than 1 mm (1/20 inch) in length. The narrow wings of thrips have fringed edges. Larvae have similar body structures but lack wings. Coloration varies among species and can range from black, brown, yellowish, or translucent.

Figure 5A. Thrips on a plant. (Photo by Frank Peairs, Colorado State University, Bugwood.org.)
Damaging Stage: Larva and adult
Damage: Damage to foliage may appear as thin white/translucent lines cutting through the foliage, yellow/brown discoloration, or brown leaf edges. Thrips can also transmit tomato spotted wilt virus, which can be detrimental to tomatoes, peppers, and eggplants.

Figure 5B. Damage from thrips on an eggplant. (Photo by J. Guyot, INRA, Pointe-à-Pitre, Bugwood.org.)
IPM Strategies:
Cultural: Managing and controlling weedy plants can prevent thrips from moving to the garden. Use yellow sticky traps to monitor for the presence of thrips.
Mechanical: Use row covers to prevent thrips from feeding on plants. Row covers are especially beneficial for young plants that are more susceptible to damage, but should be monitored to ensure that thrips have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Predatory thrips, minute pirate bugs, green lacewing larvae, predatory mites, and parasitoid wasps can help reduce pest populations. Beauveria bassiana is a commercially available pathogen that is recommended for thrips. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Bifenthrin, carbaryl, permethrin, and acephate can be used to control thrips infestations; however, these pesticides are highly toxic to beneficial insects.
Organic options: Neem oil, spinosad, and insecticidal soaps can be applied to control thrips populations. Contact sprays should be applied to the area where thrips may be present. Repeat applications may be necessary depending on the severity of pest populations.
Insects That Chew
Corn earworm/Tomato fruitworm
(Helicoverpa zea; Figures 6A and 6B)
Host: Corn is the primary host, but larvae may also feed on tomato, cotton, and sorghum. Due to its wide host range, corn earworm is also known as tomato fruitworm and sorghum headworm.
Biology and Life Cycle: Female moths lay pale green eggs on corn silk and on the upper and lower surfaces of leaves of other host plants. After three to four days, eggs will hatch, and larvae look for the reproductive structure of the plant for feeding. Once matured, larvae will leave the host plant and burrow into the soil to pupate in the summer. After ten to 25 days, adult moths emerge and become active at night. The life cycle is completed in approximately 30 days. Pupae can overwinter in the soil; however, survival decreases in temperatures below 30°F.
Metamorphosis: Complete
Overwintering Stage: Pupa
ID Tips: Larvae coloration varies, but typically the head is orange to light brown. Most species have a dark stripe located above the spiracles and a lighter stripe below the spiracles on larvae. Adult moths are typically 4 cm (1 1/2 inch) across, with shades of buff/tan and a dark-colored pattern on the forewing resembling a comma.

Figure 6A. Corn earworm larva damaging kernels of corn (Zea mays). (Photo by Eugene E. Nelson, Bugwood.org.)
Damaging Stage: Larva
Damage: Larvae bore into corn ears and eat kernels. Larvae also bore holes into tomato fruit and can cause damage to stems and leaves. Crops, such as beans, squash, and pumpkins, are also susceptible to injury, but larvae will choose silking corn over other host plants.

Figure 6B. Corn earworm adult. (Photo by Miranda Kersten, NMSU.)
IPM Strategies:
Cultural: Trap crops, such as lima beans and tomatoes, can lure moths away from their target host. Select varieties of plants that are resistant to corn earworm. Planting earlier in the season can also help reduce the likelihood of pest outbreaks.
Biological: Trichogramma wasps target corn earworm eggs, while braconid wasps attack the larval stage. Corn earworms have many predators, including damsel bugs, minute pirate bugs, big-eyed bugs, lady beetles (both the larval and adult stage), and lacewing larvae. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Monitor to determine if populations are at levels that require intervention. Use a black light, pheromone trap, or a combination of techniques to monitor relative densities and emergence times. Black-light traps capture both male and female moths, while only males are attracted to pheromone traps, but both can disrupt mating activity.
Organic options: Neem oil and Bt var. kurstaki are recommended to control corn earworm.
Cucumber beetles
(Figures 7A–7C) Spotted cucumber beetle (Diabrotica undecimpunctata) Striped cucumber beetle (Acalymma trivittatum)
Host: Cucurbits, including cucumbers, pumpkins, and summer squash, as well as corn and beans.
Biology and Life Cycle: Overwintering adults disperse to seedlings or transplants. Eggs are then deposited in the soil around the base of the plant. Larvae feed on the roots of the host plant for several weeks before pupating and emerging as adults. Typically, two generations of cucumber beetles occur in a season.
Metamorphosis: Complete
Overwintering Stage: Adult
ID Tips: Cucumber beetles are 6 mm (1/4 inch) in length and have yellow bodies. Striped cucumber beetles have defined black stripes with a black underbelly. The spotted cucumber beetle is yellow-green and has twelve black spots on its elytra.

Figure 7A. Spotted cucumber beetle on a milkweed. (Photo by Miranda Kersten, NMSU.)
Damaging Stage: Larva and adult
Damage: Adults primarily feed on foliage, pollen, and flowers of host plants, causing the undersurface of the leaf to be consumed while the upper surface is left intact. Damage to plant roots and stems by larval feeding is often minimal. Cucumber beetles may cause cosmetic damage to the rind of the fruit. Cucumber beetles serve as a vector for the bacterium Erwinia tracheiphila, which causes bacterial wilt. The bacterium is transmitted through feeding and can lead to severe losses in crop yield. This bacterium only affects cucumbers, squash, muskmelon, pumpkins, and gourds. Signs of bacterial wilt include wilting of vines and yellowing of leaves, followed by complete withering of the plant. Monitor your plants for bacterial wilt by breaking a plant stem in half and looking for stringy strands as seen in Figure 7C. When signs of bacterial wilt are detected, remove infested material—distressed plants will attract cucumber beetles and can further the spread of damage.
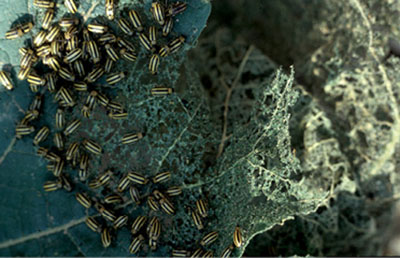
Figure 7B. Striped cucumber beetle feeding on squash plant. (Photo by Whitney Cranshaw, Colorado State University, Bugwood.org.)
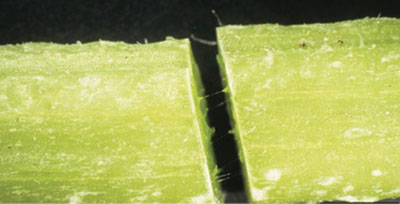
Figure 7C. Slimy strings stretching between cut stems is a sign of bacterial wilt. (Photo by Gerald Holmes, California Polytechnic State University at San Luis Obispo, Bugwood.org.)
IPM Strategies:
Cultural: Practice good sanitation and remove plants that are showing signs of bacterial wilt to reduce the transmission of the disease. Plant bacterial wilt-resistant varieties of cucurbits and use transplants to reduce vulnerability to cucumber beetles. Rotate crops each year to disrupt or reduce colonization rates of overwintering adult beetles. For large areas of crops, plant trap crops, such as zucchini and ‘Blue Hubbard’ squash, around the perimeter to lure colonizing cucumber beetles from the cash crop. Intercropping can also be an effective strategy to reduce the number of cucumber beetle populations moving into crops. Recommended intercrops include corn, broccoli, radish, cowpea, and sweet clover.
Mechanical: Use floating row covers, but remove covers when plants are flowering to allow for pollination services. Be sure to monitor under the row covers to ensure that pests have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Ground beetles, rove beetles, spiders, and predatory spider mites prey on cucumber beetle populations. Tachinid flies and braconid parasitoid wasps can reduce populations as well. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size. Apply a straw mulch to increase habitat for predators such as spiders.
Chemical: Carbaryl, pyrethrins, and permethrin can be applied to reduce cucumber beetle populations, but are extremely harmful to beneficial insects. Chemical applications can be applied to trap crops to avoid applications on desired crops.
Organic options: Sprinkle plants with kaolin clay to create a film barrier on the plant, which irritates and dehydrates insects and discourages feeding. Spinosad can also be used to control populations.
Cutworm larvae
(Figures 8A and 8B)
Host: Cutworms are generalists; some hosts plants include asparagus, beans, corn, cabbage, carrot, lettuce, peas, peppers, potato, tomato, and rhubarb.
Biology and Life Cycle: “Cutworms” refers to the larval stage of night-flying moths in the family Noctuidae. Moths deposit hundreds of eggs on the soil, plant residue, and low-growing plants, typically on weedy hosts. Eggs can be either in small clusters or laid individually. Larvae hatch and begin feeding on weeds until other plant resources like vegetables become available. Larvae hide in plant debris during the day and feed at night. Moths mate and lay eggs from early spring to early fall, resulting in up to three generations per year. Larvae survive through fall and winter by feeding on weeds until other hosts are available in the spring.
Metamorphosis: Complete
Overwintering Stage: Larva and pupa
ID Tips: Larvae grow to 5 cm (2 inches) in length and have a range of colors from brown/tan to pale pink, green, and black/gray. If disturbed, larvae will curl into a “C” shape, which is the most common identifier. Adult moths range in color from shades of brown, black, and gray. Front wings are patterned and are darker than back wings.

Figure 8A. Cutworm larvae. (Photo by Mellene Pablo, NMSU.)

Figure 8B. Adult cutworm moth. (Photo by Miranda Kersten, NMSU.)
Damaging Stage: Larva
Damage: Larvae feed on leaves and small roots until they reach 12 mm (1/2 inch) in length. The majority of damage occurs in the spring while plants are tender and have new growth. Cutworm larvae often girdle the plant at the soil level. Cutworm damage is most severe in the spring, although they are still active in the summer.
IPM Strategies:
Cultural: Remove winter weeds to reduce overwintering habitat.
Mechanical: Monitor plants for damage. If there are signs of feeding—for example, plants are cut off near the ground or showing signs of wilt—disturb the soil within a 1-foot radius to uncover cutworms. Remove cutworm larvae and kill by crushing or dropping them into soapy water. Place barriers, such as aluminum foil or cardboard collars, directly around the plant stem or create buffers of dry soil around the garden edge to discourage cutworms from entering the garden.
Biological: Cutworms have many natural enemies, including predaceous beetles, tachinid flies, parasitic nematodes, and pathogens. Ichneumonid, braconid, chalcid, and sphecid wasps play a large role in regulating cutworm populations. Mammals, birds, and reptiles also feed on cutworms. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: If cutworm damage becomes a severe problem, pesticide applications should be targeted on stems and foliage in the evening before cutworms are actively feeding. Active ingredients of commonly available pesticides include carbaryl, cyfluthrin, and permethrin; however, these pesticides are highly toxic to beneficial insects.
Organic options: Spinosad and Bt var. kurstaki can be applied to plants to reduce cutworm larvae populations.
Flea beetles
(Figures 9A and 9B)
Host: Host plant varies with species, but may include squash, corn, beans, cabbage, broccoli, lettuce, radishes, and potatoes.
Biology and Life Cycle: Adult flea beetles overwinter in either leaf litter, hedgerows, windbreaks, or forested areas and become active in the early spring. Females deposit eggs in small holes of roots, soil, or leaves of a variety of vegetable plants. Larvae feed on the roots of seedlings and will remain in the ground and transform into pupae. There can be up to two generations within a year.
Metamorphosis: Complete
Overwintering Stage: Adult
ID Tips: Adult flea beetles range from 1–3 mm (1/16–1/8 inch) in size, with a metallic black, blue, or gray body. When disturbed, flea beetles will jump using large back legs; the appearance of these legs and mode of escape gives them a resemblance to a flea.

Figure 9A. Flea beetle found on bean plant (Phaseolus vulgaris). (Photo by Howard F. Schwartz, Colorado State University, Bugwood.org.)
Damaging Stage: Larva and adult
Damage: Damage is highest during the spring. Signs of feeding resemble a shotgun-shot pattern (holes are usually less than 3 mm [1/8 inch] in diameter; Figure 9B) on the leaf of host plants; this pattern is unique to flea beetles. This type of damage can kill young plants and may reduce plant vigor. Flea beetles prefer young, tender leaves and will typically avoid older plants. Belowground feeding by flea beetle larvae can cause damage to the roots of seedlings and transplants.

Figure 9B. Flea beetle feeding damage on Chinese cabbage (Brassica pekinensis). (Photo by Howard F. Schwartz, Colorado State University, Bugwood.org.)
IPM Strategies:
Cultural: Keep plants well-watered and fertilized. Use transplants when possible. Plants grown from seed are more susceptible to damage from feeding than transplants. Remove plant debris (including weeds) to reduce overwintering habitat for adults. Alter planting schedule earlier or later in the growing season, if possible, to minimize damage. Grow a trap crop to lure pests away from desired crops. It is recommended to plant the trap crop before desired crops to serve as the main food source for the pest. Trap crops are more effective when used alongside other cultural practices.
Mechanical: Monitor populations using yellow sticky traps to determine whether you have flea beetles. If populations are identified, use floating row covers to protect plants. Be sure to monitor under the row covers to ensure that pests have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked. Remove covers during time of flowering for plants that require pollination to fruit.
Biological: Braconid wasps (specifically Microctonus vittatae) and nematodes can help to control flea beetle populations. Lacewing larvae, big-eyed bugs, and damsel bugs can also help control populations. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Populations can be difficult to control with pesticides. Using a trap crop such as radish to congregate beetle populations before applying pesticides can improve efficacy. Active ingredients of commonly available pesticides include pyrethrins/pyrethrum, carbaryl, malathion, permethrin, lambda cyhalothrin, and cyfluthrin. Keep in mind that these pesticides are highly toxic to beneficial insects.
Organic options: Use spinosad to control populations.
Grasshoppers
(Figure 10)
Host: Broad range of host plants, including trees, shrubs, grasses, flowers, and crops.
Biology and Life Cycle: In late summer and early fall, grasshoppers lay eggs in a pod-like structure in the soil. Females are able to lay up to 400 eggs within a season. The following year, nymphs emerge, becoming winged adults five to six weeks later. Although life cycles vary among species, this is the most common life cycle. Grasshoppers undergo one generation per year.
Metamorphosis: Incomplete
Overwintering Stage: Egg, nymph, and adult.
ID Tips: Bodies range in size from 2–8 cm (1–3 inches) long, and come in a variety of shapes and colors. Grasshoppers are able to fly and/or jump. Nymphs resemble adults but lack wings.

Figure 10. Grasshopper perched on a flowering plant. (Photo by Miranda Kersten, NMSU.)
Damaging Stage: Nymph and adult
Damage: Adults and nymphs feed on vegetation, which can lead to defoliation, fruit loss, and plant death. Large populations can cause severe damage and may be difficult to control.
IPM Strategies:
Cultural: Control summer weeds to reduce food sources for nymphs and remove hiding places for grasshoppers. Plant a border of trap crops, such as sunflowers or tall grasses, to minimize damage on vegetable crops. Do not mow the trap crop.
Mechanical: Floating row covers can be utilized to protect plants from grasshopper feeding. Be sure to monitor under the row covers to ensure that pests have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Robber flies, praying mantids, ground beetle larvae, bee fly larvae, and nematodes are natural enemies of grasshopper adults and nymphs. Blister beetle larvae prey on grasshopper egg pods over the winter. Apply pathogens such as Nosema locustae or Beauveria bassiana to control grasshopper populations. Nosema locustae has a 30–40% mortality rate under the best conditions, but its effectiveness decreases at low doses, when grasshoppers are large, and under high temperatures. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Applications of acephate or permethrin can be used to control populations and provide seven to ten days of control; however, these pesticides are highly toxic to beneficial insects. Chemical control should be directly applied to the nymph grasshopper stages.
Hornworms
(Figures 11A–11D) Tobacco hornworm (Manduca sexta) Tomato hornworm (Manduca quinquemaculata)
Host: Plants from the nightshade family (Solanaceae) are the primary hosts. Tomato plants are the most fed on, but larvae may be seen feeding on peppers, eggplants, and potatoes.
Biology and Life Cycle: Moths emerge from overwintering pupae in the spring and deposit light green eggs on the upper surfaces of leaves. After hatching, caterpillars begin feeding on plant material for three to four weeks, and usually remain on the same host plant, typically until the fifth instar or until food resources become scarce. Caterpillars then burrow into the soil and transform into pupae, which emerge as moths two weeks later. The second generation continues the life cycle, and pupates through the winter in the soil.

Figure 11A. Tomato hornworm larva on a garden tomato stem (Solanum lycopersicum). (Photo by Whitney Cranshaw, Colorado State University, Bugwood.org.)

Figure 11B. Tobacco hornworm larva on an ornamental chile plant (Capsicum sp.). (Photo by Marisa Thompson, NMSU.)
Metamorphosis: Complete
Overwintering Stage: Pupa
ID Tips: Tomato hornworm larvae are 7–10 cm (3–4 inches) long, with bright green coloration, V-shaped yellow-white stripes outlined in green along the side of the body, and a black/blue horn-like tail (Figure 11A). Tobacco hornworm larvae are also 7–10 cm (3–4 inches) long, but have diagonal yellow-white stripes, outlined in black along the side of the body, and have a red horn-like tail (Figure 11B). Adult moths of both species have large bodies and wings spanning 10–12 cm (4–5 inches); however, tomato hornworm adults have five orange dots on the abdomen, while tobacco hornworm adults have six orange spots on the body. Adult moths are commonly referred to as sphinx or hawk moths.

Figure 11C. Tomato hornworm adult moth perched on a leaf. (Photo by Jim Occi, BugPics, Bugwood.org.)
Damaging Stage: Larva
Damage: Caterpillars chew on leaves and stems of host plants. Fruit may also experience damage from feeding. Caterpillars produce visible dark green and black droppings (frass). Caterpillars feed extensively and can quickly defoliate plants. Feeding increases as caterpillars grow.

Figure 11D. Parasitized tomato hornworm larva covered in cotton-like cocoons from braconid wasps. (Photo by Robert L. Anderson, USDA Forest Service, Bugwood.org.)
IPM Strategies:
Cultural: Remove weeds to reduce egg-laying habitat. Rotate crops each year to disrupt pest habitat.
Mechanical: Remove caterpillars by hand and drop into soapy water. Use row covers to keep caterpillars from defoliating plants and to discourage egg laying. Be sure to monitor under the row covers to ensure that pests have not made it past the row cover barrier, where they will be protected from natural enemies and their populations can grow unchecked.
Biological: Natural enemies can help control populations. Lady beetles (both the larval and adult stage) and green lacewing larvae feed on eggs and young caterpillars. Paper wasps and braconid wasps target the caterpillar stage. Parasitized caterpillars will be covered in cotton-like cocoons (Figure 11D). If this is observed, leave the caterpillar so that adult wasps can emerge. Trichogramma wasps parasitize the hornworm eggs. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Carbaryl, permethrin, and bifenthrin can be used as a chemical control for tomato hornworms and tobacco hornworms and are best applied when newly hatched larvae are detected; however, these pesticides are highly toxic to beneficial insects.
Organic options: Bt var. kurstaki, spinosad, and insecticidal soaps can be used to suppress tomato hornworm populations. Applications are most effective against newly hatched larvae.
Insects That Harm Fruits
Codling moth
(Cydia pomonella; Figures 12A and 12B)
Host: Apple, pear, and quince.
Biology and Life Cycle: Adults emerge in the spring, typically around bloom time. A few days after emergence, moths mate and begin laying translucent eggs the size of a pinhead on fruits and leaves. Six to 14 days later, eggs hatch and larvae burrow into the fruit. They tunnel until they reach the seeds, where they will feed. Larvae go through five instars before full-grown larvae will tunnel out of the fruit and spin a cocoon in debris or beneath the bark of the tree. Evidence of burrowing includes a sting (entry point) with a red circle around the hole. Larvae that go through this process in late spring and early summer will give rise to a second generation, while larvae present in late summer will overwinter and pupate the following spring.
Metamorphosis: Complete
Overwintering Stage: Larva
ID Tips: Adults are 12 mm (1/2 inch) long, with gray/tan coloration and small alternating bands of white on the wings. Male codling moths have shiny, copper-colored scales at the ends of their wings. Larvae are 12 mm (1/2 inch) in length, with a yellow/tan body and a dark head.

Figure 12A. Codling moth adult. (Photo by Whitney Cranshaw, Colorado State University, Bugwood.org.)
Damaging Stage: Larva
Damage: Larvae bore into fruit and feed on seeds, rendering the fruit unsuitable for eating. Fruit may rot because boring can introduce fungi and/or bacteria.

Figure 12B. Damage from late-stage codling moth larva. (Photo by Whitney Cranshaw, Colorado State University, Bugwood.org.)
IPM Strategies:
Cultural: Keep areas clean by removing and destroying both fallen and infested fruit. Monitor for stings and other signs of damage. Thin fruits to prevent fruits from touching each other—this is a common point of entry for larvae. Codling moths are able to disperse up to a mile, so sanitation practices may not be enough to control populations.
Mechanical: Although labor-intensive, bagging fruits can be an effective way to prevent damage. Fruits should be covered when they are 13–25 mm (1/2–1 inch) in diameter. Trunk banding can also be an effective strategy to reduce codling moth populations. Band the trunk with sticky bands, cardboard, or burlap to trap larvae, and frequently monitor the traps. Use a high-pressure stream of water or a soap and water solution to dislodge and destroy pests, or remove banding material and destroy.
Biological: Minute pirate bugs, green lacewing larvae, spiders, and ground beetles are natural enemies of codling moths. Trichogramma, ichneumonid, and braconid wasps use codling moth caterpillars as a host. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Pheromone traps can be used to monitor codling moth flight activity, which helps determine the appropriate time to apply pesticides. Recommended pesticides include carbaryl and pyrethroids, and these should be applied to fruit after first flight has been observed. Broad-spectrum applications such as these are highly toxic to beneficial insects and should only be applied when flowers are no longer present. If more than one pesticide application is needed, consider alternating to spinosad to minimize harm to beneficial insects.
Organic options: Use pheromone traps to disrupt mating. Place pheromone traps around the area to confuse males and reduce the likelihood of males finding females. Pheromone traps have been shown to be less effective in small orchards (5 acres or less). Horticultural oils can be used to prevent eggs from hatching and should be applied before eggs hatch. Spinosad can effectively reduce populations and should be sprayed three times for spring generations (when stings are found) or twice for summer generations—all at ten- to 14-day intervals.
Lygus bugs
(Lygus hesperus and Lygus elisus; Figure 13)
Host: Apple, apricot, nectarine, peach, and pear, as well as other crops, including strawberries, tomatoes, and alfalfa.
Biology and Life Cycle: Adults emerge in the early spring and begin feeding on buds of trees and shrubs. Females will lay eggs in the stems, leaves, and buds of vegetation below the tree canopy. Three to four overlapping generations are produced in a season. Lygus bugs are also known as “plant bugs” because they feed on many types of plants and cover crops, or nearby weeds can harbor them.
Metamorphosis: Incomplete
Overwintering Stage: Adult
ID Tips: Adult lygus bugs are 5–7 mm (1/4 inch) in length, with a pale green or brown coloration and red, yellow, and black markings. These markings make an upside-down triangle centered on the upper portion of the back. Nymph lygus bugs look similar to adults but lack wings and are typically pale green.

Figure 13. Lygus bug adult on a flower. (Photo by Miranda Kersten, NMSU.)
Damaging Stage: Nymph and adult
Damage: Damage occurs from feeding on developing flower buds, fruit, and seeds. Injured stone fruits typically exhibit raised and recessed areas on the surface of the fruit. Stings (entry hole in the fruit with a surrounding red ring) that are present may have gummosis (a gummy ooze) hanging from the fruit. Additionally, fruit damage (referred to as catface injuries) results in a distorted appearance to the fruit and typically occurs during late-season feeding.
IPM Strategies:
Cultural: Remove understory weeds, such as sweet clover, lambsquarters, pigweed, shepherd’s purse, wild mustard, and vetch, to reduce egg-laying habitat.
Biological: Parasitoid wasps can attack all stages of lygus bug development. Big-eyed bugs, damsel bugs, assassin bugs, and spiders also prey on lygus bugs. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Pyrethroids can be used to control pest populations; however, repeated applications may lead to chemical resistance. These pesticides are highly toxic to beneficial insects.
Peachtree borer
(Synanthedon exitiosa; Figures 14A–14C)
Host: Prunus spp. (peach, plum, apricot, cherry, and other stone fruits).
Biology and Life Cycle: During July and August, moths lay eggs on the bark of the lower trunk or in cracks within the soil. Once the eggs hatch after approximately ten days, larvae tunnel through wound openings or cracks in the bark and begin feeding on the sapwood of trees until the arrival of cold weather. The majority of this feeding occurs at or below the ground on the trunk and on larger roots. The larvae survive the winter underground and resume feeding with the return of warmer temperatures. During mid- to late spring, the larvae mature and pupate just below the soil surface and emerge as adults about a month later, continuing the cycle.

Figure 14A. Peachtree borer found inside a peach tree (Prunus persica). (Photo by Whitney Cranshaw, Colorado State University, Bugwood.org.)
Metamorphosis: Complete
Overwintering Stage: Larva
ID Tips: Adults are about 3 cm (1 1/4 inch) in size, with a wasp-like body and clear wings. Adults are active during the daytime. Larval stage is referred to as a “borer.”

Figure 14B. Male and female peachtree borer moths (female on right). (Photo by Joseph Berger, Bugwood.org.)
Damaging Stage: Larva
Damage: Damage to the trunk, lower limbs, and roots is caused by larvae feeding on the bark of host trees and may result in tree death. One sign of borer activity is balls of sap, often mixed with frass, called gummosis, found along the trunk or around the base of an infected tree.

Figure 14C. Gummosis found along the trunk of Prunus sp. (Photo by Marisa Thompson, NMSU.)
IPM Strategies:
Cultural: Avoid mulching up to the base of the tree. Avoid tree damage from weed eaters or mowers. Maintain tree health through proper watering, pruning, and fertilization. Stressed trees are more susceptible to damage from borers.
Biological: Parasitoid wasps (Braconidae, Eulophidae, and Ichneumonidae families) attack the egg, larval, and pupal stages. Predators include ants, green lacewing larvae, and spiders. Natural enemies will not immediately eliminate the infestation and will require time to effectively reduce the population size.
Chemical: Applications of pesticides are preventive only and target the eggs and early larval stage. Once larvae have entered the tree, insecticides are no longer a viable option. Monitor adult moth activity by placing pheromone traps on trees before moths begin to fly (suggested timing is usually in May, just after petal fall). Adult moth activity helps determine when egg laying occurs (typically late June and early July)—this is when to apply chemical controls. Eggs hatch nine to ten days after moths emerge. Insecticides should have residual activity to kill emerging larvae. Permethrin or carbaryl are recommended for control of peachtree borer; however, these pesticides are highly toxic to beneficial insects.
Organic options: Neem oil can be applied to the base of the trunk to suppress eggs and prevent early larval stages from developing and boring into the tree.
Natural Enemies
Natural enemies can effectively keep pest populations in check. By incorporating floral resources throughout the growing season, you can encourage natural enemies to inhabit your garden. Below are examples of natural enemies that may be present. For more information about identifying and encouraging populations of natural enemies, see NMSU Extension Guide H-172, Backyard Beneficial Insects of New Mexico (https://pubs.nmsu.edu/_h/H172/); Guide H-169, Using Insectary Plants to Attract and Sustain Beneficial Insects for Biological Pest Control (https://pubs.nmsu.edu/_h/H169/); and the NMSU Extension publication Pocket Guide to the Beneficial Insects of New Mexico (https://pubs.nmsu.edu/insects/docs/Beneficial_Insects). Figures 15–24 are examples of natural enemies found in New Mexico.

Figure 15. Parasitoid wasps target the egg, larval, or pupal stage of their insect hosts. Their sizes range from 1–10 mm (1/25–2/5 inch). Figure 15 is a parasitoid wasp from the family Ichneumonidae. (Photo by Miranda Kersten, NMSU.)

Figure 16. Tachinid flies are parasitoids that typically attack the larval stages of their hosts. (Photo by Miranda Kersten, NMSU.)

Figure 17. A thread-waisted wasp from the Sphecidae family. These wasps are predatory at both the immature and adult stages. (Photo by Miranda Kersten, NMSU.)

Figure 18. A wasp from the Vespidae family. These wasps are predatory at both the immature and adult stages. (Photo by Miranda Kersten, NMSU.)

Figure 19. An adult syrphid fly feeding on nectar and pollen. (Photo by Miranda Kersten, NMSU.)

Figure 20. An immature syrphid fly (larval stage) preying on an oleander aphid. (Photo by David Cappaert, Bugwood.org.)

Figure 21. Both the adult and immature stage of lady beetles act as predators. The larval stage ranges in size from 1–8 mm (1/25–3/10 inch). (Photo by Miranda Kersten, NMSU.)

Figure 22. Adult lacewings typically feed on pollen and nectar; however, they do occasionally act as predators. Their size ranges from 8–25 mm (3/10–1 inch). (Photo by Miranda Kersten, NMSU.)

Figure 23. The larval stage of lacewings preys on many soft-bodied insect hosts. Their size reaches up to 12 mm (1/2 inch). (Photo by
Miranda Kersten, NMSU.)

Figure 24. Both the adult and immature stages of minute pirate bugs act as predators. Their size ranges from 2–3 mm (2/25–3/25 inch), and they can be difficult to see. (Photo by Miranda Kersten, NMSU.)
Glossary
Beneficial insects are insects that perform ecosystem services, such as pollination and pest suppression. This includes pollinators and natural enemies (predators, parasitoids, and pathogens).
Complete metamorphosis is the developmental process in which an insect undergoes four life stages—egg, larva, pupa, and adult. Each stage looks distinctly different from the previous stage.
Cornicles are the protruding, tube-like structures on the abdomen of aphids (Figure 1B).
Elytra are the wing cases of a beetle.
Frass is the excrement from insects.
Gummosis occurs from sap oozing from wounds or cankers on fruit trees and resembles a gummy substance. Typically, this is caused by insect problems, climatic conditions, infections, or mechanical damage (Figure 14C).
Incomplete metamorphosis is the developmental process in which an insect undergoes three life stages—egg, nymph, and adult. The nymph and adult stages resemble each other. Adults are typically larger than nymphs and may have wings.
Instar refers to either larval or nymph growth stages that are separated by molting.
Natural enemies are predators, parasitoids, and pathogens that are beneficial because they reduce the numbers of pest insects by killing them or limiting their reproductive success.
Ovipositor is the tubular organ, attached to the abdomen of the insect, that is used to lay eggs.
Parasitoids are insects whose larval stage lives within an insect host. Eventually, the larval stage emerges and kills the host, and the adult stage will lay eggs on or within a new host insect.
Predators are insects or other arthropods that feed on multiple prey items throughout their life. Many of these predators would be considered beneficial natural enemies.
Pupa is a developmental stage in insects that undergo complete metamorphosis, and is the point where insects will transform from the immature to the mature stage of their life cycle.
Secondary pest outbreaks occur when chemical applications aimed to reduce the population of one pest species trigger a subsequent outbreak of a different pest population. Often the result of management methods that wipe out natural enemy populations.
Spiracles are the pores on the body of an insect that aid in respiration.
Stings are the entry holes in fruit with a surrounding red ring made by larvae tunneling into fruit.
Viral vector refers to insects that transmit viruses to plants through feeding.
Resources
Plant diagnostic services are available for New Mexico residents through the NMSU Plant Diagnostic Clinic (https://aces.nmsu.edu/ces/plantclinic/). Bring samples to the nearest NMSU Cooperative Extension Service office to be sent to the NMSU Plant Diagnostic Clinic for free services, including analysis of plant material for pathogens, pests, and environmental stresses. Find your local Extension office at https://aces.nmsu.edu/county/, and learn more about submitting plant samples in NMSU Extension Guide H-158, How to Collect and Send Plant Specimens for Disease Diagnosis (https://pubs.nmsu.edu_h/H158/).
Online sources for ordering organic control options and beneficial insects include Arbico Organics (www.arbico-organics.com), Planet Natural Garden Supply (www.planetnatural.com), and Beneficial Insectary, Inc. (www.insectary.com). Contact your local plant nursery to see what options are available in stores.
NOTE: These sources are for resource use only and are not meant as an endorsement by New Mexico State University or the funding sources of this publication.
References
Adam, K.L. 2006. Squash bug and squash vine borer: Organic controls [ATTRA Publication IP298]. National Center for Appropriate Technology. Retrieved April 8, 2019, from https://attra.ncat.org/product/squash-bug-and-squash-vine-borer-organic-controls/
Alston, D., and M. Murray. 2010, November. Greater peachtree borer (Synanthedon exitiosa) [ENT-103-07]. Logan: Utah State University Cooperative Extension and Utah Plant Pest Diagnostic Laboratory.
Archer, T.L., and E.D. Bynum, Jr. 1994. Corn earworm (Lepidoptera: Noctuidae) biology on food corn on the High Plains. Environmental Entomology, 23, 343–348. https://doi.org/10.1093/ee/23.2.343
Bethke, J.A., S.H. Dreistadt, and L.G. Varela. 2014. Thrips: Integrated pest management for home gardeners and landscape professionals [Publication 7429]. Berkley: University of California Cooperative Extension.
Bunn, B., D. Alston, and M. Murray. 2015, April. Flea beetles on vegetables (Coleoptera: Chrysomelidae) [ENT-174-15]. Logan: Utah State University Cooperative Extension and Utah Plant Pest Diagnostic Laboratory.
Cornell University Plant Disease Diagnostic Clinic. 2019. Bacterial wilt of cucurbits: Erwinia tracheiphila. Ithaca, NY.
Cranshaw, W. 2004. Garden insects of North America: The ultimate guide to backyard bugs. Princeton, NJ: Princeton University Press.
Cranshaw, W.S., and R. Hammon. 2013. Grasshopper control in gardens and small acreages [5.536]. Fort Collins: Colorado State University Extension.
Day, E.R. 2014. Aphids [444-220]. Blacksburg: Virginia Cooperative Extension.
Delahaut, K. 2005. Squash bug [XHT1135]. Madison: University of Wisconsin Extension.
Delahaut, K.A., E.M. Cullen, and J.L. Wedberg. 2004. Corn earworm [A3655]. Madison: University of Wisconsin Extension.
Ellington, J., T. Carrillo, J. White, C. Sutherland, D. Richman, J. Drake, and S. Bundy. 2005. Guide to the biological control of some common yard and garden pest insects in New Mexico [Circular 607]. Las Cruces: New Mexico State University Cooperative Extension Service.
Foster, R.E., and J. Obermeyer. Managing insects in the home vegetable garden [E-21-W]. West Lafayette, IN: Purdue University Extension Entomology.
Hahn, J.D., M. Grabowski, and J. MacKenzie. 2011. Pest management for the home apple orchard [H-15]. St. Paul: University of Minnesota Extension.
Harding, J.A. 1976. Heliothis spp.: Seasonal occurrence, hosts and host importance in the lower Rio Grande Valley. Environmental Entomology, 5, 666–668.
Hein, G.L., J.B. Campbell, and R.C. Seymour. 2006. A guide to grasshopper control in yards and gardens [G1633]. Lincoln: University of Nebraska-Lincoln Extension.
LeVeen, E., and A.C. Hodges. 2014. Bagrada bug, painted bug, Bagrada hilaris (Burmeister) (Insecta: Hemiptera: Pentatomidae) [EENY596]. Gainesville: University of Florida Extension.
McKinlay, R.G. 1992. Vegetable crop pests. Boston: CRC Press.
Munyaneza, J.E. 2003. Leafhopper identification and biology. In Pacific Northwest Vegetable Association Proceedings (pp. 89–91).
Palmer, M.A. 1952. Aphids of the Rocky Mountain Region. Thomas Say Foundation.
Palumbo, J.C. 2000. Management of aphids and thrips on leafy vegetables. Tucson: University of Arizona Cooperative Extension.
Parker, J.E., C. Miles, and W.E. Snyder. 2012. Organic management of flea beetles [PNW640]. Pullman: Washington State University Extension.
Peairs, F.B., and J.L. Capinera. 2002. Caterpillars on field crops: III [5.565]. Fort Collins: Colorado State University Extension.
Reed, D.A., T.M. Perring, J.P. Newman, J.A. Bethke, and J.N. Kabashima. 2014. Bagrada bug [74166]. Berkley: University of California Cooperative Extension.
Snyder, W.E. 2019. Managing cucumber beetles in organic farming systems [64274]. Pullman: Washington State University Extension.
Waters, T.D., and C.H. Wohleb. 2016. Corn earworm pest of sweet corn [FS221E]. Pullman: Washington State University Extension.
Wilen, C.A., D.L. Haver, M.L. Flint, P.M. Geisel, and C.L. Unruh. 2006. Pesticides: Safe and effective use in the home and landscape [74126]. Oakland: University of California.
Wold-Burkness, S., and J. Hahn. 2007. Tomato hornworms in home gardens [M1224]. St. Paul: University of Minnesota Extension.
Wyman, J.A., and P.J. Pellitteri. 1998. Managing insects in the home vegetable garden [A2088]. Madison: University of Wisconsin Extension.
For Further Reading
H-169: Using Insectary Plants to Attract and Sustain Beneficial Insects for Biological Pest Control
https://pubs.nmsu.edu/_h/H169/
H-172: Backyard Beneficial Insects of New Mexico
https://pubs.nmsu.edu/_h/H172/
H-174: Integrated Pest Management (IPM) Strategies for Common Insect Pests of Trees in New Mexico
https://pubs.nmsu.edu/_h/H174/

Miranda Kersten is a Senior Program Specialist with the urban integrated pest management (IPM) program at NMSU’s Agricultural Science Center in Los Lunas. Her work focuses on pollinator and beneficial insect conservation, monitoring beneficial insects across urban landscapes, and managing IPM research projects.
This work is supported by the Crop Protection and Pest Management Program (grant no. 2017- 70006-27189) project accession no. 1013838 from the National Institute of Food and Agriculture.
The pesticide recommendations in this publication are provided only as a guide. The authors and New Mexico State University assume no liability resulting from their use. Please be aware that pesticide labels and registration can change at any time; by law, it is the applicator’s responsibility to use pesticides ONLY according to the directions on the current label. Use pesticides selectively and carefully and follow recommended procedures for the safe storage and disposal of surplus pesticides and containers.
Brand names appearing in publications are for product identification purposes only. No endorsement is intended, nor is criticism implied of similar products not mentioned. Persons using such products assume responsibility for their use in accordance with current label directions of the manufacturer.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu.
Contents of publications may be freely reproduced, with an appropriate scitation, for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
August 2021, Las Cruces, NM


