Guide H-174
Ashley B. Bennett , Miranda L. Kersten, Marisa Y. Thompson
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Respectively, Former Small Urban Farm IPM Specialist , Department of Extension Plant Sciences; Senior Program Specialist , Agricultural Science Center at Los Lunas; Urban Horticulture Specialist, Department of Extension Plant Sciences. (Print Friendly PDF)
Table of Contents
Integrated Pest Management for Urban Trees
Integrated Pest Management for Urban Trees
Integrated pest management (IPM) is a sustainable approach to pest management that combines cultural, mechanical/physical, biological, and chemical control strategies in a way that minimizes economic, health, and environmental risks (USDA–ARS, 2018). When managing urban trees for insect pests, the following steps are key to a successful IPM program.
- Prevention – Avoid insect pests through proper plant selection, planting techniques, tree maintenance, irrigation, and fertilization.
- Monitoring – Regularly inspect your trees and shrubs for insect pests or signs of damage.
- Identification – Be sure to correctly identify both the tree species and insect pests that are present. Correct identification is critical to creating an effective IPM plan.
- Management – Select the appropriate IPM strategies for the pest, including cultural, mechanical, biological, and chemical pest suppression tactics. Below is a description of each strategy.
Cultural control strategies make the environment less suitable for pests.
Examples:
- Select tree species/cultivars that are stress-resistant or tolerant (stressors include pests, drought, disease, and heat or cold).
- Select a proper site for trees and shrubs in terms of soil, light, space, wind, and water.
- Keeping trees and shrubs healthy and minimizing stress can prevent pests.
- Over-watering can kill tree roots, encourage weeds, and promote disease.
- Under-watering leads to drought stress and increases susceptibility to some pests.
- Sanitation involves removing plant debris from the landscape to reduce overwintering habitat for pest species.
- Stand management will improve tree vigor and reduce the dispersal capabilities of pests.
Mechanical/physical tactics use equipment or barriers to protect plants.
Examples:
- Hand removal of insect pests, such as bagworm, can be effective but labor-intensive.
- Trapping can be used to monitor for pests as well as reduce low-level pest populations.
- Pruning can remove localized infestations on leaves and branches.
- A high-pressure stream of water can dislodge insects feeding on foliage or dislodge egg cases.
- A protective coating of paint on trunks can reduce sunscald (aka winter injury) and the associated open wounds, thereby reducing the chance of attack from wood-boring insects.
- Sticky barriers applied to tree trunks can prevent crawling pests from reaching the upper parts of a tree.
Biological control methods use living organisms (natural enemies) to suppress pest populations.
Two types of biological controls can be implemented in urban landscapes: augmentation and conservation. Augmentation biological control directly manipulates the natural enemy community through releases of insect predators or parasitoids. Many insect predators and parasitoids are available commercially and can be released to enhance natural pest suppression. Conservation biological control manipulates the habitat to favor existing natural enemy populations. Incorporating certain flowering plants (floral resources) into the landscape can attract natural enemies by providing food and nesting resources. Many natural enemies feed on nectar, such as parasitoids, or supplement their diet with pollen, like lady beetles. Unfortunately, urban landscapes often lack the floral resources needed to support natural enemies. Floral resources are needed from early spring to late fall to support and build effective natural enemy populations. Early season resources are often lacking in urban landscapes, but incorporating flowering trees and shrubs into the landscape can help fill this resource gap. Natural enemies also need nesting and overwintering habitat, so leave some amount of perennial plants, leaves, and fallen logs to provide these resources. Chemical controls often have detrimental effects on natural enemy populations, so avoid or limit the use of chemicals to protect natural pest suppression provided by natural enemies. For more information on using floral resources to attract natural enemies, see NMSU Extension Guide H-169, Using Insectary Plants to Attract and Sustain Beneficial Insects for Biological Pest Control (https://pubs.nmsu.edu/_h/H169.pdf).
Chemical control uses synthetic as well as naturally derived compounds that kill, repel, regulate, or interrupt the growth of insect pests.
IPM strives to eliminate or reduce the use of chemical controls, especially in urban landscapes where human health risks are high. When practicing IPM for urban trees and shrubs, use cultural, mechanical, and biological controls before trying chemical controls. Specific chemicals are not recommended in this guide because product registrations change over time. If chemical controls are needed, follow these best management practices:
- Correctly identify the pest to select the best chemical control.
- Always read and follow chemical label instructions carefully.
- Apply chemical control during the correct life stage because some insect life stages will be more or less susceptible to insecticides.
- Apply chemical controls under the recommended wind speeds and temperatures.
- To slow the development of insecticide resistance, do not spray the same chemical consecutively, avoid using the same chemical family, and use the application rate on the label.
- Do not spray when trees or shrubs are blooming to protect beneficial insects like bees.
- Select chemical controls that do not persist in the environment.
- Use narrow-spectrum chemicals that target the pest but reduce impacts on natural enemies, e.g., Bt (Bacillus thuringiensis).
- Select chemical controls, such as horticultural oils and insecticidal soaps, that limit non-target effects on natural enemies and other beneficial insects.
- Contact your county Extension agent (https://aces.nmsu.edu/county/) to find products appropriate for your insect pest.
Using This Guide
This guide will help you identify insect tree pests and select successful IPM strategies. Terms that have been bolded are defined in the glossary, which can be found toward the end of this guide.
Each insect pest has been assigned a damage level: aesthetic, reduction of vigor, or mortality. Aesthetic damage reduces the plant’s appearance but generally does not harm it. Reduction of vigor refers to damage that negatively impacts tree health, causes death to limbs, and may impact reproductive success of the individual tree. Insect pests that reduce tree vigor may also damage the aesthetics of a tree or shrub. Some insect infestations will result in the death of the tree, and those are listed as mortality.
Defoliators
Bagworm (Thyridopteryx ephemeraeformis; Figures 1A–1C)
Host: Conifers and deciduous trees, such as arborvitae, pine, locust,
sycamore, and oak.
Biology and life cycle: Adults mate in the fall, with females laying eggs in bags they have created out of foliage and silk. The eggs hatch in the spring, and larvae crawl out of the bags and begin feeding on foliage. The length of the larval stage is temperature-dependent and can last up to four months if conditions are favorable. Bagworms produce one generation per year and overwinter in the egg stage.
Metamorphosis: Complete
Overwintering stage: Egg
ID tips: Bagworm presence is easily recognized by their foliage-constructed bags hanging from branches. Bags range in size from 30–75 mm (1–3 inches).
Damage: Larvae cause defoliation of host trees.
Damage level: Aesthetic
IPM Strategies
- Cultural: Keep trees healthy with proper watering and nutrient management so trees can tolerate bagworm feeding.
- Mechanical: Remove bags by hand when infestations are low. Once bags are removed, destroy them completely to prevent eggs from hatching and returning to the tree. Bags can be burned, sealed tightly in trash bags, or drowned in soapy water. The best time of year for hand removal is late fall to early spring, before eggs hatch.
- Biological: Encourage natural enemies by promoting flowering species in the area. Bagworms have parasitoids, such as ichneumon and torymid wasps, which require nectar resources as adults.
- Chemical: Contact insecticides, such as insecticidal soaps, are most effective when larvae are young.
- Organic options: Small larvae can be controlled with Bt var. kurstaki or spinosad. Entomopathogenic nematodes can control larvae if applied under favorable environmental conditions; their effectiveness declines with hot and dry conditions. Traps baited with a sex pheromone attract and trap males, which disrupts mating and can reduce populations over time. Check with local nurseries about these options.
Figure 1A. A bagworm bag hangs from a partially defoliated leaf (photo by Ashley B. Bennett, New Mexico State University).
Figure 1B. A bagworm in the pupal stage (photo by Ashley B. Bennett, New Mexico State University).
Figure 1C. Bagworm bags (photo by Marisa Thompson, New Mexico State University).
Cottonwood leaf beetle (Chrysomela scripta; Figure 2)
Host: Cottonwood, aspen, willow, and alder.
Biology and life cycle: Adults overwinter under bark and in leaf litter on the ground. Females lay yellow, elongated eggs in clusters of 15–75 on the underside of leaves. These eggs hatch after one to two weeks. Larvae then spend one to two weeks feeding on leaves before pupating on branches and leaves. After one to two weeks in the pupal stage, adults emerge. There are at least two generations per year; however, this number may vary based on climate and latitude.
Metamorphosis: Complete
Overwintering stage: Adult
ID tips: Cottonwood leaf beetle larvae resemble grubs and can grow to a length of 12 mm (1/2 inch). Young larvae are black in color, while older larvae become brownish or gray with white spots along their abdomen. Adult beetles are approximately 6 mm (1/4 inch) in length, yellowish in color, and have two front and back black spots. Each side of the elytra has two longitudinal black stripes.
Damage: Larvae and adults both feed on leaves. Larvae feed in groups, often on the underside of the leaf, causing skeletonization. Adult feeding results in holes and eventual loss of entire leaves.
Damage level: Aesthetic, reduction of vigor
IPM Strategies
- Cultural: Remove adult overwintering sites by removing weeds and leaf litter from around trees before winter. Tree cultivars with greater pubescence have demonstrated some resistance to damage.
- Biological: Insect predators of cottonwood leaf beetles include predatory stink bugs, lady beetles (e.g., Coleomegilla maculata), ants, spiders, and parasitoids, such as pteromalid wasps and tachinid flies. These predators can help control eggs and larvae.
- Chemical: Pesticides, such as acephate and carbaryl, can be used to control cottonwood leaf beetle larvae and adults. Please check for current recommended application levels before use.
- Organic options: Young larvae can be controlled with organic insecticides, such as neem oil, spinosad, and Bt var. San Diego and tenebrionis, but these may not be effective for mature larvae and adults.
Figure 2. Cottonwood leaf beetles emerge from their pupae (photo by Ashley B. Bennett, New Mexico State University).
Elm leaf beetle (Xanthogaleruca luteola; Figures 3A and 3B)
Host: Elm
Biology and life cycle: Females lay eggs in double rows of 5–25 eggs on the underside of leaves. These eggs can be yellowish or gray colored. When larvae first hatch, they are gray but turn yellowish or greenish as they mature. Larvae feed on leaves for several days and then crawl to the base of the tree to pupate. Adult beetles emerge in 7–10 days and feed on leaves. There can be one to three generations per year, depending on climate. Adults overwinter in crevices on the tree, leaf litter, and woodpiles. Elm leaf beetle populations fluctuate dramatically from year to year. Few if any elms require treatment every year.
Metamorphosis: Complete
Overwintering stage: Adult
ID tips: Adult elm leaf beetles are an olive green color and have two black stripes along the outside edge of their back. Elm leaf beetles are about 6 mm (1/4 inch) long.
Damage: Larvae and adults both feed on leaves. Larvae feed in groups, often on the surface of the leaf, which results in skeletonization. Adults chew holes in leaves, and eventually cause the loss of entire leaves. Large infestations of elm leaf beetles can defoliate trees.
Damage level: Aesthetic, but can cause reduction of vigor.
IPM Strategies
- Cultural: Healthy elms can withstand defoliation by elm leaf beetles. To keep elms healthy, irrigate trees as needed.
- Biological: Tachinid flies attack the larval stage of the elm leaf beetle, while eulophid wasps attack the egg and pupal stages. Both of these parasitoids can be encouraged through floral resource plantings.
- Chemical: Bark band trees by applying a contact insecticide around the lower trunk of the tree to treat late-instar larvae as they crawl down the tree to pupate in the soil. The band should surround the entire tree and cover several feet of the tree trunk.
- Organic options: Spinosad can be applied to the foliage to target early instar larvae.
Figure 3A. An adult elm leaf beetle rests near elm leaf beetle eggs (photo by Ashley B. Bennett, New Mexico State University).
Figure 3B. An elm leaf beetle larva feeds on an elm leaf (photo by Ashley B. Bennett, New Mexico State University).
Fall webworm (Hyphantria cunea; Figures 4A–4C)
Host: Deciduous trees, including pecan, walnut, mulberry, sycamore, alder, willow, oak, cottonwood, and fruit trees.
Biology and life cycle: Female moths lay clusters of several hundred yellow eggs on the underside of leaves. Eggs hatch after one to two weeks. Caterpillars live in groups in webbed nests at the end of tree branches. Nests look similar to those of tent caterpillars (Malacosoma californicum). Caterpillars pupate in soil and under loose bark after about six weeks of feeding. They overwinter as pupae, with adults emerging in the late spring or early summer. Adults are nocturnal and attracted to light. Fall webworms produce one to two generations per year, with more generations possible in warmer climates.
Metamorphosis: Complete
Overwintering stage: Pupa
ID tips: Caterpillars create silk webs on the end of tree limbs. Fall webworm caterpillars grow up to 25 mm (1 inch) long. Caterpillars are hairy and vary in coloration, from yellow to dark gray with yellow to dark-colored spots and cream-colored stripes along their sides. The wings of adult moths are white and may have dark spots, depending on location. They have a wingspan ranging from 25–50 mm (1–2 inches).
Damage: Caterpillars cause defoliation by feeding on leaves inside their webs. Webs are expanded as foliage becomes depleted and access to new leaves is needed.
Damage level: Aesthetic
IPM Strategies
- Cultural: Keep your trees healthy so they can tolerate webworm infestations.
- Mechanical: Webs can be knocked off with a high-pressure water sprayer or broken open with a stick or broom handle. Freed caterpillars can then be trampled.
- Biological: Parasitoid wasps (families Braconidae, Ichneumonidae, Eulophidae, Pteromalidae, and Trichogrammatidae) and tachinid flies attack eggs and larvae. Birds and spiders also feed on fall webworm caterpillars. Opening webs helps these natural enemies gain access to caterpillars.
- Chemical: Insecticides are often not recommended since trees can tolerate low to moderate fall webworm damage. However, if used, insecticides should be applied to webs (inside webs, if possible) and surrounding areas when caterpillars are small.
- Organic options: Bt and spinosad can be applied to the web with a high-pressure sprayer soon after caterpillars have hatched.
Figure 4A. A web created by fall webworms covers a tree branch (photo by Ashley B. Bennett, New Mexico State University).
Figure 4B. Fall webworm caterpillars feed on a leaf inside their web (photo by Ashley B. Bennett, New Mexico State University).
Figure 4C. Fall webworm caterpillars feed on a leaf (photo by Ashley B. Bennett, New Mexico State University).
Leaf miners (Figure 5)
Examples of leaf miner pests in New Mexico include pecan serpentine leaf miner (Stigmella juglandifoliella) and aspen leaf miner (Phyllocnistis populiella).
Host: Ornamental trees and shrubs, pecan.
Biology and life cycle: Eggs are laid on the underside of leaves. After hatching, larvae tunnel into the leaf and begin feeding on plant tissue inside the leaves, creating mines that are especially visible when held up to light. Larvae pupate in the soil or leaf litter. Adults emerge in the early spring.
Metamorphosis: Complete
Overwintering stage: Pupa
ID tips: Opening up the mined area exposes a hollow area in the leaf where the insects and/or frass may be present.
Damage: Larval feeding creates winding mines or tunnels within leaves. Mined areas of foliage are often lighter in color due to feeding, and these leaves may drop prematurely. Leaf miner damage is generally more of an aesthetic issue than harmful to the plant.
Damage level: Aesthetic
IPM Strategies
- Cultural: Keep trees healthy with proper watering and nutrient management.
- Mechanical: Remove and destroy infested leaves. If leaf miners have infested trees locally, cover nearby trees and shrubs with cheesecloth or fine screen to prevent access by dispersing adults.
- Biological: Most leaf miners are suppressed by natural enemies, including parasitoid wasps such as eulophid wasps (Diglyphus spp.), pteromalid wasps, and braconid wasps.
- Chemical: Insecticides are often not recommended since trees can tolerate low to moderate damage. Contact insecticides can be applied prior to egg laying, which is generally shortly after leaves emerge in the spring.
- Organic options: Neem oil and spinosad can be effective for treating leaf miners.
Figure 5. Leaf miner damage on aspen in the Carson National Forest (photo by Marisa Thompson, New Mexico State University).
Pine sawfly (Neodiprion spp.; Figures 6A and 6B)
Host: Conifers, including ponderosa pine, piñon pine, true fir, Douglas fir, hemlock, and spruce.
Biology and life cycle: Female adults have a saw-like ovipositor to cut slits into the tree’s needles, where they lay eggs. Eggs are laid in rows in slits in the needles, and they overwinter in this stage. Larvae emerge in the spring and feed on older needles. Larvae form a pupal case composed of a thin, cylindrical cocoon in leaf litter or soil, or under tree bark. Adults emerge in late summer/early fall. Pine sawflies usually produce one generation per year.
Metamorphosis: Complete
Overwintering stage: Egg
ID tips: The larvae resemble caterpillars and are about 10 mm (2/5 inch) in length, with three pairs of true legs and six or more pairs of prolegs. Larvae feed in groups and wave their bodies when threatened. Larval color varies with species, and includes green or yellow-green bodies with black, tan, or orange heads. Adults resemble flies but have two sets of wings. Female adults are typically 10–12 mm (2/5–1/2 inch) long and yellowish-brown, and have a thick waist, while males are smaller (5–7 mm [less than 1/4 inch]) with feathery antennae.
Damage: Presence can be initially detected by looking for thinning in the tree crown since larvae feed on the older needles. Defoliation by larvae progresses each year, slowing tree growth. Defoliation weakens trees, leaving them susceptible to attacks from other insects and diseases.
Damage level: Cumulative, but infestation of small trees may result in mortality.
IPM Strategies
- Cultural: Stressed plants (water- and/or nutrient-limited) are more susceptible to infestations. Discourage sawfly introduction with supplemental watering during times of drought and through proper nutrient management.
- Mechanical: For localized infestations, remove by hand or prune out infected branches. Knock larvae off by shaking branches or by spraying with a high-pressure water sprayer. Be sure to kill larvae by crushing them or drowning them in soapy water.
- Biological: Natural enemies targeting larvae include birds, rodents, and insect predators. Rodents eat pupae.
- Chemical: Large infestations may require chemical controls.
- Organic options: Insecticidal soaps and horticultural oils; application should target young larvae. Note that Bt does not control sawflies.
Figure 6A. A group of pine sawfly caterpillars feeds on pine needles (photo by Ashley B. Bennett, New Mexico State University).
Figure 6B. Pine sawfly caterpillars after a molt (photo by Ashley B. Bennett, New Mexico State University).
Pine tip moths (Figures 7A–7C)
Eight species of pine tip moths occur in New Mexico, including Nantucket pine tip moth (Rhyacionia frustrana), southwestern pine tip moth (Rhyacionia neomexicana), and the moths in genus Dioryctria that attack piñon pine.
Host: Yellow pine, ponderosa pine, piñon pine, Afghan pine, Douglas fir, arborvitae, Scotch pine, and mugo pine.
Biology and life cycle: Eggs are laid on terminal buds and on new shoots. Larvae bore into new needle shoots and feed through July. Pine tip moth larvae activity begins with bud break, but the timing varies with each pine tip moth species. Mature larvae move to the base of trees where they form a silken cocoon and pupate. Depending on the species, they overwinter as pupae or larvae in the soil, in stems, or under tree bark. Most pine tip moths produce one generation per year, but the Nantucket pine tip moth may produce two to three generations per year in New Mexico.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Adults are about 19 mm (3/4 inch) long with mottled wings that range in color from yellowish gray to rusty brown. Larvae are yellow to red-orange in color with black heads and are about 13 mm (1/2 inch) in length.
Damage: Pine tip moth larvae cause damage by boring into new needle growth in the spring and summer, which can cause stem deformity. Feeding results in dieback of the branch tips; small trees are more susceptible to injury.
Damage level: Aesthetic, but can reduce vigor in small trees and trees under 12 feet.
IPM Strategies
- Cultural: Maintain healthy trees through proper watering and nutrient management.
- Mechanical: Prune back branches infested with pine tip moths.
- Biological: Predators include ants, spiders, mites, and parasitoids, including eulophid and eurytomid wasps and tachinid flies. Mice and birds may also feed on pine tip moths.
- Chemical: Proper timing of chemical control is critical and varies by species. Apply chemicals, such as acephate or carbaryl, to branch tips after eggs are laid but before larvae have hatched.
- Organic options: Apply Bt to branch tips to control young larvae. Pheromone traps used in conjunction with sticky traps can be used to trap and monitor certain species of pine tip moths.
Figure 7A. A pine tip moth caterpillar and related feeding damage (photo by Ashley B. Bennett, New Mexico State University).
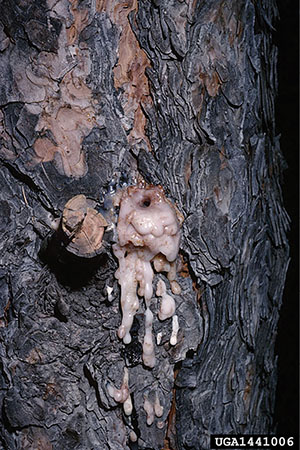
Figure 7B. Damage to a pine tree from pine tip moth feeding (photo by Ashley B. Bennett, New Mexico State University).
Figure 7C. Sap on a tree branch from pine tip moth damage (photo by Ashley B. Bennett, New Mexico State University).
Western tent caterpillar (Malacosoma californicum; Figures 8A and 8B)
Host: Deciduous trees, including fruit trees, aspen, and willow.
Biology and life cycle: Western tent caterpillars overwinter in egg masses attached to host trees. Egg masses have up to 400 eggs and are covered in spumaline, a brownish-gray substance that provides protection through the winter. Emergence from eggs is timed with bud break of host trees. Larvae create a dense mass of silk for protection, but leave their silken tents to feed at night. Larvae are active for seven to eight weeks, and by late spring are mature. Larvae spin a cocoon to pupate and remain in the pupal stage for about two weeks. Adults emerge and mate, with females laying one egg mass in summer. Tent caterpillars produce one generation per year.
Metamorphosis: Complete
Overwintering stage: Egg
ID tips: Look for silk tents in the crotches of tree branches. Fall webworms (Hyphantria cunea) are more prominent in the fall, and their webs occur at the ends of tree branches. Larvae are 40–50 mm (1 1/2–2 inches) long, hairy, and a pale blue-gray color with a light-colored stripe and blueish spots running down the middle of their backs. Adults are 25–38 mm (1–1 1/2 inches) long and rusty orange to light yellow or tan in color.
Damage: Tent caterpillars are defoliators, and heavy defoliation may result in branch dieback. Extended outbreaks of more than three years may cause tree mortality, but outbreaks usually last only two to three years.
Damage level: Aesthetic, but will reduce tree vigor after several years of defoliation.
IPM Strategies
- Cultural: Keep outdoor lights turned off at night when possible to avoid attracting moths to ornamental/landscape trees. Proper water and nutrient management keeps trees healthy and able to tolerate defoliation.
- Mechanical: Remove egg masses in the fall and winter. Egg masses are easier to spot when the leaves have dropped from the tree. Remove tents in the spring when caterpillars are present. Use a high-pressure water sprayer to destroy tents.
- Biological: Natural enemies of tent caterpillars include birds, vespid wasps, tachinid flies, and parasitoid wasps such as egg parasitoids (family Eulophidae) and external larval parasitoids (family Braconidae). A nuclear polyhedrosis virus (NPV) can also infect larvae.
- Chemical: Localized chemical applications to tents containing caterpillars can be effective.
- Organic options: Bt and spinosad can be used to control western tent caterpillars.
Figure 8A. Western tent caterpillars (photo by John Formby, New Mexico State Forestry).
Figure 8B. Western tent caterpillars create webs in the crotches of tree branches (photo by Brytten Steed, USDA Forest Service, Bugwood.org).
Sap Feeders
Aphids (Figures 9A–9C)
Examples of aphids in New Mexico include cottonwood leaf aphids, pine aphids, oak aphids, and woolly aphids.
Host: Most plants can be hosts for aphids, with some aphids using different hosts for winter and summer. Winter hosts may include fruit trees, roses, cottonwood, willow, and dogwood. Preferred summer hosts include plum, roses, hawthorn, crape myrtle, apple, and willow. Examples of conifer hosts include pine, fir, and spruce.
Biology and life cycle: Most aphids reproduce asexually, with female aphids giving birth to live nymphs. Aphids undergo incomplete metamorphosis, meaning immature and adult aphids look similar with the exception of size. Immature aphids, or nymphs, typically progress through four nymphal stages before becoming an adult. In the fall, some species produce sexual morphs, which can be winged, that mate and lay eggs on a winter host. Winged aphids can also be produced early in the season and when aphid populations reach levels where food resources become limited. Aphids produce multiple generations per year.
Metamorphosis: Incomplete
Overwintering stage: Egg
ID tips: Nymphs are small and wingless, and resemble adults. Adult aphids are small, soft-bodied, and pear-shaped, usually without wings. Both adult and immature aphids of most species have cornicles, tube-like structures projecting from the posterior end of their bodies. Adults range in size from 1.5–8 mm (1/10–1/3 inch) depending on the species. Aphid colors range from colorless to green, yellow, pink, red, brown, or black.
Damage: Aphid feeding results in yellowing of foliage, wilting, distorted leaves, and leaf drop. Aphids also produce honeydew, which is a clear, sugary, sticky liquid that causes sooty mold to grow on host plants. The presence of sooty mold can reduce the photosynthetic ability of host plants. Honeydew also attracts ants that can interfere with biological control services. While most aphids feed on aboveground leaf tissue, some aphids, such as the woolly apple aphid, feed on roots.
Damage level: Aesthetic
IPM Strategies
- Cultural: Aphids flourish on new growth, so over-fertilizing plants encourages aphids.
- Mechanical: On smaller plants with low infestations, knock aphids off leaves by using a high-pressure stream of water, or kill aphids by hand. Because ants help protect aphids, Tanglefoot or sticky tape applied to the bases of trees inhibits access by ants.
- Biological: Insect predators include lady beetles (adults and larvae), syrphid flies (larvae), small spiders, lacewings (larvae), and minute pirate bugs. Parasitoids from the families Braconidae and Aphelinidae commonly attack aphids. A parasitized aphid is called a mummy and appears brown or gray and swollen.
- Chemical: Aphids can be treated with a variety of conventional insecticides.
- Organic options: Dormant oils can be used to kill eggs. Insecticidal soaps and summer oils (e.g., horticultural oils and neem) kill adults by suffocation, so good coverage is necessary. Do not apply soaps or oils to drought-stressed plants or when temperatures rise above 90°F because plants can be damaged.
Figure 9A. Aphids come in many colors. Oleander aphids (Aphis nerii) are a bright gold, and are shown here with a lady beetle. (Photo by Ashley B. Bennett, New Mexico State University).
Figure 9B. Aphids may also be a dark gray color. (Photo by Ashley B. Bennett, New Mexico State University).
Figure 9C. Parasitized aphids, also called mummies, appear swollen and gray. (Photo by Ashley B. Bennett, New Mexico State University).
Ash whitefly (Siphoninus phillyreae; Figures 10A–10C)
Host: Ornamental trees, fruit trees, and ash.
Biology and life cycle: Pale yellow, waxy eggs are laid on the underside of leaves. Nymphs are opaque and become covered in white wax as they develop. Ash whiteflies develop a “pupal case” that has a longitudinal waxy strip running down the middle and tubercles around the edges that produce clear liquid droplets. Adults have light-colored bodies with white wings. Adult females are around 1–2 mm (1/15 inch) in size and live from 30–60 days. Adult males live for about nine days. While whiteflies feed on deciduous hosts, they tend to overwinter on evergreens. Several generations per year are possible, with warmer climates having more generations.
Metamorphosis: Incomplete
Overwintering stage: All stages
ID tips: Adults resemble small moths with white wings that have a waxy appearance. Ovipositing females leave waxy white circles on leaves surrounding eggs.
Damage: Nymphs and adults feed on leaves, causing wilting and leaf curling at the tips. Heavy feeding can cause leaf drop and loss of smaller fruits in fruit-bearing trees. Whiteflies secrete honeydew that can cause the growth of sooty mold.
Damage level: Aesthetic unless populations are high, then a reduction in vigor can occur.
IPM Strategies
- Cultural: Because ash whiteflies are greenhouse pests with a broad host range that includes ornamental trees, check for ash whitefly presence on nursery stock before planting. Removing overwintering evergreen hosts, especially when diseased and in decline, can reduce ash whitefly populations. Reflective mulches can repel whiteflies, but this strategy is most effective with smaller plants.
- Mechanical: Remove infested leaves. Use a high-pressure stream of water to dislodge adults from plants. Depending on the population density, pruning branches may cause more damage than the insects.
- Biological: Natural enemies include parasitoid wasps and lady beetles. The California Department of Food and Agriculture developed a classical biological control program for this pest, releasing the imported parasitoid, Encarsia spp., and a lady beetle, Clitostethus arcuatus. In California, both of these natural enemies effectively control ash whitefly populations. Other lady beetles, parasitoid wasps from the family Aphelinidae, and generalist predators, such as green lacewings, big-eyed bugs, and minute pirate bugs, attack ash whitefly. In New Mexico, promote natural enemies by incorporating flowers into your landscape for food and nesting resources.
- Chemical: Because ash whiteflies are typically on the underside of leaves, contact chemicals are often not effective. Systemic insecticides are not recommended due to negative impacts on natural enemies and bees.
- Organic options: Insecticidal soaps, neem oil, and petroleum-based oils are effective but require direct contact with the pest.
Figure 10A. Ash whiteflies look like tiny moths but are actually true bugs (photo by Ashley B. Bennett, New Mexico State University).
Figure 10B. Female ash whiteflies leave a circular white residue on the leaf (photo by Ashley B. Bennett, New Mexico State University).
Figure 10C. The ash whitefly nymph and adult stages all present on one leaf. Parasitoid wasps (one pictured in the center of this photo) are an important biological control measure for ash whiteflies (photo by Ashley B. Bennett, New Mexico State University).
Mealybugs (Figure 11)
Obscure mealybug (Pseudococcus viburni) is common on woody trees and shrubs in New Mexico.
Host: Fruit and nut trees
Biology and life cycle: Eggs hatch into crawlers (nymph stage) in about 10 days. Crawlers range in color from yellow, pinkish, or orangeish; lack wax; and are mobile. Crawlers disperse to other plant parts and feed for four to eight weeks until they become adults. Once they begin feeding, a waxy covering develops over their bodies. Mealybugs can overwinter as eggs or young crawlers. They can produce up to six generations per year, with females laying 100–200 eggs in a wax-covered egg mass.
Metamorphosis: Incomplete
Overwintering stage: Egg or young crawler
ID tips: Presence of mealybugs can be detected by looking for the white waxy covering on older nymphs and adults. Visible mealybugs are often female and found in aggregations. Adult female mealybugs are oblong in shape, segmented, and wingless, while adult males have two wings. Mealybugs are quite small, around 1–4 mm (1/25–3/20 inch) long.
Damage: Mealybugs (nymph and adult stages) feed on plant phloem. Feeding can cause yellowing of foliage, wilting, leaf drop, and twig dieback. Mealybugs secrete honeydew while feeding, and black sooty mold can develop on leaves, reducing the plant’s ability to photosynthesize.
IPM Strategies
- Cultural: Keep plants healthy with proper watering and nutrient management. Sanitizing work tools is important and prevents inadvertently transferring mealybugs to uninfested plants. When planting new trees and shrubs, inspect plant material for mealybugs to avoid introductions.
- Mechanical: With low infestations, mealybugs can be removed by hand or pruning out infested branches. Mealybugs can also be washed off plants using a high-pressure stream of water.
- Biological: Promote natural enemies of mealybug, such as lady beetles, green lacewings, minute pirate bugs, spiders, and parasitoid wasps in the families Aphelinidae and Encyrtidae. The mealybug destroyer (Cryptolaemus montrouzieri) is lady beetle that is an important predator of mealybugs. Ants can transport mealybugs between plants, so control ants to slow mealybug movement.
- Chemical: Chemical controls should target the crawlers, which are the most susceptible stage.
- Organic options: Neem oil and insecticidal soaps can be used to control the crawler stage.
Figure 11. An adult mealybug destroyer feeds on a mealybug (photo by Palex66, Dreamstime.com).
Two-spotted spider mite (Tetranychus urticae; Figures 12A and 12B)
Host: Maple, elm, redbud, roses, arborvitae, elm, and fruit and nut trees such as pear and walnut.
Biology and life cycle: Spider mites overwinter as adults at the base of host plants in leaf litter or under bark. In the spring, eggs are laid on the underside of leaves. They hatch into larvae that then undergo two nymphal stages before becoming adults. Adults have piercing mouth parts that allow them to suck sap from leaves. Larvae have six legs, while nymphs and adults have eight legs. Spider mite development is temperature-dependent; their life cycle can take one to three weeks to complete. Spider mites thrive in hot, dusty environments.
Metamorphosis: Incomplete
Overwintering stage: Adult
ID tips: Spider mites are quite small (0.4 mm; 1/60 inch) and difficult to see without a hand lens (10–15× magnification). Check for tiny moving dots on the underside of leaves as well as the presence of webbing on leaves. Mites have an oval body shape with two dark spots on the front of their body.
Damage: Spider mites typically cause yellow stippling on leaves, but when infestations are high, they can cause necrotic spots on leaves and leaf drop. Feeding on flowers can cause browning and withering of petals.
Damage level: Aesthetic
IPM Strategies
- Cultural: Mite outbreaks generally occur in dry and dusty conditions. Keep trees well watered, and dust off tree foliage and fruits.
- Mechanical: Use a strong stream of water to wash dust off trees. Pruning out branches with localized infestations of mites may cause more damage than the insects.
- Biological: Important natural enemies of spider mites include predatory mites (e.g., Phytoseiulus spp.), predatory thrips, lacewing larvae, minute pirate bugs, big-eyed bugs, and spider mite destroyer lady beetles (Stethorus punctum).
- Chemical: Miticide selection is important because spider mites have developed resistance to some products. Most miticides do not kill eggs.
- Organic options: Insecticidal soaps and horticultural oils can be used to control spider mites. Apply when mites first appear.
Figure 12A. Two-spotted spider mite (photo by David Cappaert, Bugwood.org).
Figure 12B. Leaf damage caused by two-spotted spider mites (photo by Whitney Cranshaw, Colorado State University, Bugwood.org).
Spittlebugs (Figures 13A and 13B)
Examples of spittlebugs in New Mexico include pine spittlebug (Aphrophora cribrata) and pecan spittlebug (Clastoptera achatina).
Host: Oak, juniper, arborvitae, pine, sage, aspen, dogwood, and alder.
Biology and life cycle: Spittlebugs spend the winter as eggs laid in the bark of twigs and branches. In the spring, nymphs emerge and feed on plant sap in the xylem tissue. Nymphs secrete a foamy fluid to keep their bodies moist and protect themselves from predators. Adults are present in the summer and continue to feed on plant fluids. Spittlebugs produce one generation per year.
Metamorphosis: Incomplete
Overwintering stage: Egg
ID tips: Adult spittlebugs resemble leafhoppers. Size and coloration vary among species, but they are typically brown in color and approximately 6 mm (1/4 inch) in size. Nymphs can be found by looking for the frothy white fluid covering their bodies.
Damage: Nymphs and adult stages feed on plant juices, but cause little to no damage to trees. Large infestations can cause leaf distortion, stunting, and yellowing of infested leaves and needles.
Damage level: Aesthetic
IPM Strategies
- Cultural: Nymphs often die from desiccation during high temperatures. Consider removing weeds infested with spittlebugs in the spring to prevent dispersal to woody trees and shrubs.
- Mechanical: Spittlebug nymphs can be removed from trees using a high-pressure stream of water or by pruning out branches with spittlebugs, although more damage may be caused by pruning than the spittlebugs.
- Biological: Encourage natural enemies, including ants, spiders, and birds.
- Chemical: Infestations usually are not high enough to warrant chemical controls.
- Organic options: Insecticidal soaps can be used if applied thoroughly.
Figure 13A. Pine spittlebug nymph and the frothy fluid that indicates the presence of spittlebugs (photo by Lacy L. Hyche, Auburn University, Bugwood.org).
Figure 13B. Adult pecan spittlebug (photo by Louis Tedders, USDA Agricultural Research Service, Bugwood.org).
Scale Insects
Armored scale insects (Figures 14A and 14B)
Armored scales in New Mexico include San Jose scale (Quadraspidiotus perniciosus), oystershell scale (Lepidosaphes ulmi), and euonymus scale (Unaspis euonymi).
Host: Elm, oak, apple, roses, crabapple, willow, ash, cotoneaster, pine, and many other fruit and shade trees.
Biology and life cycle: Crawlers are the first-instar nymphs that hatch from eggs and disperse across plants before settling down to feed on plant fluids. Scale insects have piercing-sucking mouthparts, and they tap into the plant cells to feed. Once crawlers settle and begin feeding, they become immobile and develop a hard waxy protective covering. Female scales are wingless and become legless during later nymphal stages. Males have one pair of wings, and most die after mating in the spring. The number of generations per year varies by species, from one to several.
Metamorphosis: Incomplete
Overwintering stage: Varies by species
ID tips: Armored scales produce a waxy covering for protection and are circular or oblong in shape. Color varies by species and can be black, brown, tan, or white. Scales appear as raised bumps on bark, stems, or conifer needles. Armored scales range in size from approximately 3–13 mm (1/8–1/2 inch).
Damage: Feeding on leaf tissue causes yellowing and browning in deciduous leaves, as well as needle drop in pines. Oystershell scales feed on plant cells in the bark, infesting branches and the base of trees. San Jose scale can infest foliage as well as fruit (e.g., apple, pear, peach, and plum). Heavy scale populations can weaken trees, promoting pathogens, branch dieback, and tree mortality. Scale insects produce honeydew, causing the growth of black sooty mold.
Damage level: Aesthetic, reduction of vigor
IPM Strategies
- Cultural: Control ants. Ants protect scale insects from natural enemies. To reduce ants, place baits or sticky tape on the base of trees to inhibit ant access to the tree.
- Mechanical: Use a high-pressure stream of water to remove scale insects from vegetation. Rake up downed plant material and burn it. Prune off infested branches and discard infested material. Oystershell scale can be removed from bark by gently scrubbing the bark with a brush or scrubbing pad.
- Biological: Promote natural enemies, which include lady beetles, lacewings, and parasitoid wasps. Parasitoids in the family Aphelinidae commonly attack pine needle scale and San Jose scale. Some birds, such as nuthatches and brown creepers, feed on San Jose scale.
- Chemical: Insecticides and insecticidal soaps can be used, but application should target crawlers. Avoid using broad-spectrum insecticides, which also kill beneficial insects.
- Organic options: Horticultural oils may help control scale insects. Dormant oils can be applied during the winter season to target overwintering eggs. Summer oils kill young scales, crawlers, and eggs. Caution: Do not apply oils to new growth or trees under stress. Oil application can discolor spruce trees.
Figure 14A. Oystershell scales on an aspen (photo by Whitney Cranshaw, Colorado State University, Bugwood.org).
Figure 14B. San Jose scales (photo by Takumasa Kondo, University of California–Davis, Bugwood.org).
Kermes scale (Kermes spp.; Figure 15)
Host: Oak, including pin oak, Gambel oak, red oak, and bur oak.
Biology and life cycle: Kermes scale crawlers feed on tree sap through the early summer until reaching maturity. Males are very small and winged, while females are immobile. Females continue to grow through the summer as eggs mature. Eggs hatch in the fall, and first-instar nymph crawlers settle around buds on nearby branches to overwinter. When leaves emerge in the spring, crawlers either remain at these buds or move to other areas with new growth to feed. Kermes scales produce one generation per year.
Metamorphosis: Incomplete
Overwintering stage: First-instar nymph
ID tips: Female kermes scales can be found around the base of leaf stems. Female scales are rounded, brownish in color, and grow to around 6.5 mm (1/4 inch) in diameter. They can be mistaken for buds.
Damage: Damage by kermes scales is localized to the branches on which the scales are feeding. Kermes scales cause reduced growth on these branches. Trees with heavy infestations may experience leaf yellowing and leaf loss. Branches may die back or fall off the tree. Tree sap may be observed oozing from the tree. Kermes scales produce honeydew, which can promote the growth of a black sooty mold.
Damage level: Reduction of vigor
IPM Strategies
- Cultural: Stressed trees are more susceptible to infestation by kermes scales. Keep trees healthy with proper watering and nutrient management.
- Mechanical: Sticky traps can be used to capture scale insects and monitor their population. Remove kermes scales by hand or prune infected branches.
- Biological: Promote natural enemies. Lady beetles and some species of pyralid caterpillars (family Pyralidae) feed on scales. Vespid wasps may feed on eggs and mature females.
- Chemical: Insecticides and insecticidal soaps can be used, but timing of the application is important. Avoid using broad-spectrum insecticides, which also kill natural enemies and other beneficial insects. Pyrethroid insecticides can be applied in the fall to control crawlers. Systemic insecticides can be applied to the soil in the fall to help control scales the next year. Please seek out currently recommended pesticides.
- Organic options: Horticultural oils may help control scale insects if applied when crawlers are active.
Figure 15. Kermes scales on a tree branch (photo by Ashley B. Bennett, New Mexico State University).
Pinyon needle scale (Matsucoccus acalyptus; Figures 16A and 16B)
Host: Piñon pine and bristlecone pine.
Biology and life cycle: Eggs are laid in clusters at the base of the tree trunk and the underside of branches. Eggs are yellow and covered with a cottony webbing. Eggs hatch about four to six weeks after being laid, and about seven to 10 days after the red eye spots have appeared on the eggs. Once the eggs hatch, yellow nymphs (called crawlers) move onto the previous year’s needles and begin feeding. After feeding begins, the crawlers become immobile and develop a waxy covering that is black in color and bean-shaped. Females overwinter as nymphs under the protective covering. Males emerge from this immobile stage in the fall and move to the ground where they overwinter as pupae. Activity begins again in the spring when males emerge to mate with the females. White cottony egg masses are laid at the base of trees, on the underside of branches, and in cracks in the bark. Pinyon needle scales produce one generation per year.
Metamorphosis: Incomplete
Overwintering stage: Males as pupae, females as nymphs.
ID tips: Nymphs in the black bean stage are the most commonly observed because they are present from late spring through the winter. Crawlers are present in early spring. To check for their presence, hold a piece of white paper under a branch and shake the branch to dislodge the crawlers onto the paper. Adult males are winged and resemble a small fly. Males are active in early spring. Females are about 2 mm (1/15 inch) long.
Damage: Nymphs feed on older needles, causing needle drop and discoloration. Repeated feeding can cause stunted growth and deformity to the needles. Feeding damage can result in death of smaller trees when combined with drought stress.
Damage level: Reduction of vigor
IPM Strategies
- Cultural: Keep trees and shrubs healthy by watering them during times of drought to discourage pinyon needle scale infestations. Good sanitation practices, such as raking up and destroying egg masses located at the base of trees, can reduce scale populations.
- Mechanical: Dislodge egg masses from trees using a high-pressure stream of water; rake fallen debris from under and around the tree and destroy it. Removing egg masses using mechanical and cultural control can be effective in reducing scale populations.
- Biological: Effective biological control agents (e.g., predators and parasitoids) are not documented for this pest.
- Chemical: Insecticides are most effective when applied to the crawler stage or after red eye spots have appeared on the eggs. A carbaryl and dormant oil solution can offer control for pinyon needle scales.
- Organic options: Summer oils can be effective on egg masses, crawlers, and newly settled scale stages. Dormant oils can also be used to control pinyon needle scale. However, these horticultural oils should not be used on new growth or on drought-stressed plants because they can damage plants. Insecticidal soaps can be effective when the crawler stage is present.
Figure 16A. Pinyon needle scales (photo by Ashley B. Bennett, New Mexico State University).
Figure 16B. Damage from extreme pinyon needle scale infestation (photo by John Formby, New Mexico State Forestry).
Bark Beetles
Bark beetles (Figures 17A–17F)
Bark beetles in New Mexico include engravers (Ips spp.), mountain pine beetle (Dendroctonus ponderosae), western pine beetle (Dendroctonus brevicomis), twig beetles (Pityophthorus spp.), and European elm bark beetle (Scolytus multistriatus).
Host: Pine, spruce, fir, and elm.
Biology and life cycle: Adult bark beetles bore under bark and create passages in which small, white, oval-shaped eggs are laid. Larvae are also white in color. Larvae continue mining the inner bark of the tree as they feed on phloem. Larvae will pupate in the tree. Adults exit the tree and move to new trees to begin the cycle again. The life cycle of one generation can take from 30 to 40 days. Up to seven generations are possible in one year.
Metamorphosis: Complete
Overwintering stage: Larva or adult, depending on species.
ID tips: Boring creates frass, which resembles sawdust, that can be present at the base or bark crevices of infested trees. Boring also starts the flow of tree sap, creating pitch tubes. Look for small, round exit holes in the bark of trees where adults have emerged. Adults are typically not observed, but are less than 7 mm (1/3 inch) in length with a stout body shape, and are dark red, brown, or black in color.
Damage: Bark beetles tunnel under bark where they feed on the phloem and cambium layers, eventually girdling the tree. Bark beetles may also introduce pathogens, such as blue staining fungi, to host trees. Blue stain fungus clogs the water-conducting tissue (xylem), preventing water movement within the tree. Beetle feeding causes needle discoloration and branch dieback. An attack by bark beetles causes tree mortality.
Damage level: Mortality
IPM Strategies
- Cultural: Stressed trees are more susceptible to attack from bark beetles, so keep trees healthy with proper water and nutrient management. Prune during winter months when bark beetles are less active. Stack freshly cut wood away from uninfested trees. Select tree species appropriate for your location to minimize stress.
- Mechanical: With freshly cut wood, peel back bark and expose the wood to the sun to heat-treat bark beetle larvae. Cut wood can also be covered with dark plastic to increase the temperature and kill larvae (i.e., solarization).
- Biological: Natural enemies of bark beetle larvae include checkered beetles, mites, snakeflies, predatory flies, woodpeckers, and braconid, ichneumonid, and pteromalid wasps.
- Chemical: Preventive insecticides can be applied to tree trunks and limbs to kill adults. Preventive sprays may be warranted for high-value un-infested trees. However, once adults have bored into trees, chemical controls become much less effective.
- Organic options: Bark beetles locate mates using pheromones. Pheromone traps can be used to monitor for bark beetle presence, and when populations are low can help suppress localized populations. Repellent pheromone traps can be used to keep beetles away from individual trees.
Figure 17A. Adult bark beetles are not often seen but are quite small. (Photo by Ashley B. Bennett, New Mexico State University).
Figure 17B. Bark beetles create tunnels under bark. (Photo by Ashley B. Bennett, New Mexico State University).
Figure 17C. Blue stain fungi are introduced into trees by bark beetles. (Photo by Ashley B. Bennett, New Mexico State University).
Figure 17D. Exit holes from bark beetles are small and round (photo by Ashley B. Bennett, New Mexico State University).
Figure 17E. Pitch tubes from a bark beetle attack (photo by John Formby, New Mexico State Forestry).
Figure 17F. Frass from Ips spp. bark beetle boring activity (photo by John Formby, New Mexico State Forestry).
Mountain pine beetle (Dendroctonus ponderosae; Figure 18)
Host: Ponderosa and lodgepole pine; limber pine, piñon pine, Douglas fir, and spruce may occasionally be used as hosts.
Biology and life cycle: Adults are active from July through September. Female beetles lay around 75 eggs under the bark of host trees. After hatching, larvae tunnel away from the egg passages and feed on the tree phloem. The pupal stage remains under the bark until adults emerge and disperse to neighboring trees. Mountain pine beetles overwinter as larvae under the tree bark and produce one generation per year.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Look for pitch tubes on the trunk that can identify where a beetle has bored into the tree. Dust from boring activities may be found on the bark or at the base of the tree. Holes from woodpecker feeding can also indicate the presence of mountain pine beetles.
Damage: Larvae damage trees when they bore under the bark and feed on the tree phloem. Mountain pine beetles carry blue stain fungi, which helps kill the tree more quickly by blocking the water-conducting tissues within the tree. Increased woodpecker activity may lead to missing patches of bark. Mountain pine beetle infestations can result in tree mortality.
Damage level: Mortality
IPM Strategies
- Cultural: Keep trees heathy by pruning and thinning when necessary, and by avoiding water stress. Plant a variety of tree species with varying ages and sizes. Do not transport firewood because this can spread bark beetles into previously uninfested areas.
- Mechanical: Remove trees that are infested or at risk from stress, age, or injury. Burn, bury, or chip infested logs, or place infested logs under plastic for several months to kill larvae (solarization); to ensure mortality, temperatures should reach over 110°F from solar heating.
- Biological: Checkered beetles (family Cleridae) feed on the adult and larval stages, while woodpeckers target the larvae.
- Chemical: Preventive pesticides can be applied prior to infestation, but are typically reserved for high-value trees.
- Organic options: Pheromone traps can capture beetles and may reduce small, isolated populations. Pheromone traps can also be used to monitor for the presence of bark beetles.
Figure 18. A pitch tube created by mountain pine beetle activity (photo by USDA Forest Service, Region 2 – Rocky Mountain Region, Bugwood.org).
Western pine beetle (Dendroctonus brevicomis; Figure 19)
Host: Ponderosa pine
Biology and life cycle: Adult females lay small, white eggs in tunnels. These eggs hatch into larvae that feed on phloem, then pupate inside the tree. Adults emerge from underneath the tree bark and seek out new trees to infest. Western pine beetles can produce several generations per year, ranging from two to four. The number of generations depends on geographic location and length of the growing season. Western pine beetles overwinter in the larval stage.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Western pine beetle presence can be detected by looking for resin and dust that result from adult and larval boring. Removing bark reveals winding feeding passages that often cross, which is characteristic of this species. This beetle is one of the smaller bark beetles, only 3–5 mm (1/8–1/5 inch) in size, dark brown in color, and oblong in shape.
Damage: Feeding becomes evident through fading of foliage, which first turns a pale green and then yellow, orange, and reddish-brown. Adult beetles carry spores of a blue stain fungus, which blocks the water-conducting vessels of the tree. Western pine beetle damage results in tree mortality.
Damage level: Mortality
IPM Strategies
- Cultural: Keep trees healthy by watering in times of drought. Having a variety of tree species can reduce Ponderosa pine density, lowering western pine beetle infestations. Check for western pine beetle presence before transporting firewood.
- Mechanical: Remove infested trees to reduce pest dispersal to neighboring trees. If removing entire trees, kill beetle larvae by removing bark, and burning or chipping infested logs. Placing infested logs under plastic for several months can kill larvae (solarization); to ensure beetle mortality, temperatures should reach over 110°F from solar heating.
- Biological: Support natural enemies of western pine beetles, including checkered beetles, braconid and pteromalid wasps, and woodpeckers.
- Chemical: Preventive pesticides can be applied to at-risk trees prior to infestation.
- Organic options: Pheromone traps can be used for monitoring purposes and to reduce small beetle populations.
Figure 19. Winding feeding passages, or galleries, are characteristic of the western pine beetle (photo by Ladd Livingston, Idaho Department of Lands, Bugwood.org).
Wood Borers
Honey locust borer (Agrilus difficilis; Figure 20)
Host: Honey locust
Biology and life cycle: Adults are active from early summer through early fall. Females feed on tree foliage and lay eggs on tree wounds, scars, and cankers. After hatching, larvae tunnel under the bark and feed on the cambium. Honey locust borers overwinter as larvae underneath the bark or in the sapwood. They produce one generation per year.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Larvae are cream-colored, legless, and segmented. Adult beetles reach approximately 7–13 mm (1/4–1/2 inch) in size. They are a dark metallic color, with yellow bands on their abdomens. Adults create a D-shaped exit hole in the bark. Sap stains may appear on bark around infested sites.
Damage: Larvae feed on the cambium layer, creating serpentine tunnels underneath the bark. Larvae generally focus their feeding on previously damaged portions of the bark. Infestations may result in branch dieback.
Damage level: Reduction in vigor
IPM Strategies
- Cultural: Injured and stressed trees are more susceptible to attacks, so maintain healthy trees through proper water and nutrient management.
- Mechanical: Remove and destroy dying limbs, but avoid pruning when adults are active.
- Biological: Parasitoid wasps from the Braconidae family target the larval stage.
- Chemical: A contact insecticide, such as permethrin, may be applied to tree bark during the early summer when egg laying/hatching occurs. However, insecticides are not recommended as an effective treatment for this pest. Always follow chemical labels carefully.
Figure 20. Adult honey locust borer (photo by Ashley B. Bennett, New Mexico State University).
Lilac-ash borer (Podosesia syringae; Figure 21)
Host: Lilac, ash, and New Mexico olive.
Biology and life cycle: Adults emerge from the bark and are active from spring to early July. After their emergence, adult females are only active for less than two weeks. During this time, a single female can lay hundreds of eggs. Tan, oval eggs are laid singly or in small clusters in bark crevices or near wounds in the lower portion of the trunk. Eggs hatch after one to two weeks. After hatching, larvae tunnel under the bark and begin feeding on the different layers of the trunk. They overwinter as larvae within their tunnels and pupate at the end of the larval tunnel in the early spring. When adults emerge later in the spring, the pupal casing can often be seen hanging from the tree trunk. Lilac-ash borers produce one generation each year.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Adults mimic paper wasps, with yellow bands on a black body and clear wings that span from 28–35 mm (1–1 1/3 inches). They also have long brown legs similar to a paper wasp; however, they have a broader waist than that of a wasp. Exit holes are irregularly round and about 6 mm (1/4 inch) in diameter. Larvae are white, have a brown head, and can reach approximately 25 mm (1 inch) in length. Look for sawdust around the tunnel entrance that results from larval feeding.
Damage: Larvae feed in the cambium layer, creating tunnels. They may also move into the sapwood and heartwood of the tree. Larval feeding can cause leaf and branch dieback; however, larvae typically only affect trees that are already stressed or injured. Feeding may cause cankering on the tree bark.
IPM Strategies
- Cultural: Prevent infestations by keeping trees healthy. Provide supplemental watering during times of drought and avoid injuring the tree.
- Mechanical: Remove infected branches and destroy them to kill larvae. Care should be taken when pruning, and pruning should not occur when moths are active.
- Biological: Natural enemies of the larval stage include woodpeckers and parasitoid wasps from the Braconidae and Ichneumonidae families.
- Chemical: Apply contact insecticides, such as permethrin or carbaryl, shortly before eggs hatch.
- Organic options: Pheromone traps are available for clearwing moths to monitor adult activity and assist with the timing of chemical applications. Entomopathogenic nematodes can be applied to tunnel entrances in the bark to control larvae.
Figure 21. Adult lilac-ash borers mimic paper wasps, but are actually day-active moths (photo by Whitney Cranshaw, Colorado State University, Bugwood.org).
Locust borer (Megacyllene robiniae; Figures 22A and 22B)
Host: Black locust and its cultivars (e.g., purple robe locust).
Biology and life cycle: Adults lay eggs in cracks and crevices of tree bark in the fall. Larvae hatch in the fall and bore into the inner bark where they overwinter. In the spring, larvae first bore into the sapwood and then into the heartwood of the tree, completing their development in mid to late summer. Adults are active in late summer and early fall and can often be found feeding on goldenrod pollen and nectar. Locust borer beetles produce one generation per year.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Larvae are cream-colored and legless, with a brown head. Larvae grow up to 25 mm (1 inch). Adult beetles are black with bright yellow bands and reddish legs. Locust borers are long-horned beetles, and their antennae are unusually long. Adult beetles reach approximately 19 mm (3/4 inch) in size.
Damage: Larvae create deep feeding tunnels that weaken trees, making them susceptible to wind damage. Trees under stress are more susceptible to attacks. Signs of an infestation include dead and broken limbs, swollen areas on the trunk, and wood dust emerging from oval-shaped exit holes in the bark.
Damage level: Reduction in vigor
IPM Strategies
- Cultural: Black locust trees in monoculture stands are more susceptible than those in mixed stands. Consider removing infested trees to prevent dispersal to neighboring healthy trees. Stressed trees are more susceptible to attack, so maintain healthy trees through proper water and nutrient management.
- Biological: Natural enemies include woodpeckers and wheel bugs.
- Chemical: Tree trunks can be sprayed with an insecticide in mid to late summer to protect them from attack.
Figure 22A. An adult locust borer feeding on Riddell’s ragwort (Senecio riddellii) pollen (photo by Miranda L. Kersten, New Mexico State University).
Figure 22B. Adult locust borers mating above their oval-shaped exit hole (photo by Marisa Y. Thompson, New Mexico State University).
Long-horned beetles (Figures 23A–23C)
Long-horned beetles in New Mexico include spotted pine sawyer (Monochamus clamator), cottonwood borer (Plectrodera scalator), and banded ash borer (Neoclytus caprea).
Host: Maple, poplar, willow, cottonwood, ash, elm, apple, locust, and untreated lumber and firewood.
Biology and life cycle: Eggs are laid in bark, on branches, and on the trunk of trees in a pit chewed by females, with single eggs laid in the cambium layer of the tree. Larvae bore into the wood, feeding as they go deeper into the sap and heartwood of the tree. Larvae generally pupate in the spring under the bark. After about two to seven weeks, adults emerge. Adult long-horned beetles feed on flowers. While most species complete development in one year, some species take multiple years to develop. Long-horned beetles overwinter in the larval stage.
Metamorphosis: Complete
Overwintering stage: Larva
ID tips: Adults are large, ranging from 23–40 mm (1–1 1/2 inches) long, with long antennae. Colors vary by species. Beetle presence may be indicated by sawdust found at the base of trees and checking for 10 mm (1/3 inch) and larger round exit holes in the bark.
Damage: The pit created by female beetles for the eggs discharges sap and stains tree bark a red-brown color. Larvae boring results in serpentine tunnels that structurally weaken trees. Feeding damage can cause yellowing of foliage, wilting, and branch dieback.
Damage level: Reduction of vigor
IPM Strategies
- Cultural: Long-horned beetles tend to attack stressed and weakened trees, so discourage beetles by removing dead limbs and dying trees. Remove dead limbs during the winter or early spring when adults are not present. Avoid pruning when adults are actively laying eggs. Check plant material, lumber, and firewood for beetles before introducing into your property. Prevention is the best IPM strategy when managing wood-boring beetles.
- Mechanical: When infestations are low and larvae do not bore deeply into the wood, larvae can be killed by piercing their bodies with a wire inserted into the bore hole. With high infestations, consider removing and burning the tree before adults emerge. Solarization (i.e., covering with plastic) can be used to kill larvae in freshly cut wood as well as exclude adults.
- Biological: Support natural enemies of long-horned beetles, including click beetles, flat bark beetles, assassin bugs, ambush bugs, robber flies, and parasitoid wasps and flies (families Ichneumonidae, Braconidae, and Tachinidae). Birds and lizards also feed on beetles (larvae and adults).
- Chemical: Pesticides are not effective in managing most long-horned beetles. Preventive applications of pesticides to bark and limbs may be effective for some species.
Figure 23A. Cottonwood borer (photo by Jim Baker, North Carolina State University, Bugwood.org).
Figure 23B. Banded ash borer (photo by USDA Forest Service–Ogden, Bugwood.org).
Figure 23C. Pine sawyer beetle (photo by Eugene E. Nelson, Bugwood.org).
Gall Formers
Hackberry gall psyllid (Pachypsylla celtidismamma; Figures 24A–24C)
Host: American and net-leaf hackberry
Biology and life cycle: In the spring, eggs are laid on the underside of new leaves. After about 10 days, nymphs hatch from the eggs and being feeding on leaves. Feeding by the nymph causes swelling in the leaf tissue that ultimately covers the nymph and creates the gall. Gall formation takes about one to two weeks. Nymphs continue to live in these galls, feeding on the gall tissue, until they emerge as adults in the fall. The hackberry gall psyllid overwinters as an adult in the tree bark or other protected areas and produces one generation per year.
Metamorphosis: Incomplete
Overwintering stage: Adult
ID tips: Look for galls (raised bumps) on the leaves of trees. Adults look similar to a small cicada, about 5–6 mm (1/5 inch) in length and brown in color. Opening up a gall reveals the nymph inside, which lacks wings and is light cream in color.
Damage: Hackberry gall psyllids form galls on the leaves of hackberry trees. This damage is often mostly aesthetic, but can result in leaves dropping early in the growing season.
Damage level: Aesthetic
IPM Strategies
- Cultural: Healthy trees can tolerate feeding by adults and nymphs.
- Mechanical: Remove leaves with galls. Parasitoid wasps may overwinter in the galls, so it can be beneficial to leave some galls on the tree. Since parasitoid wasps infect the nymphal stage, these infected galls will not exhibit exit holes from adults in the fall. Look for exit holes in the spring to verify the presence of parasitoids.
- Biological: Parasitoid wasps are the most effective biological control agent. Wasps in the families Torymidae, Encyrtidae, and Eurytomidae are known parasitoids of this pest.
- Chemical: Pesticides are ineffective when nymphs are inside galls. Preventive applications may be helpful if applied prior to egg laying in the spring. However, damage is often only aesthetic, and chemicals are not recommended.
Figure 24A. Galls on hackberry leaves are a sign of the presence of hackberry gall psyllids (photo by Ashley B. Bennett, New Mexico State University).
Figure 24B. Feeding by hackberry gall psyllids can also cause stippling on leaves (photo by Ashley B. Bennett, New Mexico State University).
Figure 24C. A hackberry gall psyllid nymph inside a gall (photo by Ashley B. Bennett, New Mexico State University).
Natural Enemies
To help conserve and increase natural enemies, add floral resources to the landscape and make sure pollen and nectar are available throughout the growing season. Figures 25–31 show examples of natural enemies that are commonly found.
Figure 25. Parasitoid wasps come from the superfamilies Ichneumonoidea and Chalcidoidea, among others. The immature stage is predaceous, while adults visit flowers for nectar. Parasitoid wasps may infect the egg, larval, or pupal stage of their insect hosts. Parasitoid wasps range in size from approximately 1–10 mm (1/25–2/5 inch) (photo by Miranda L. Kersten, New Mexico State University).
Figure 26A. A predatory wasp from the Sphecidae family. The immature and adult stages are predaceous (photo by Ashley B. Bennett, New Mexico State University).
Figure 26B. A predatory wasp from the Vespidae family. The immature and adult stages are predaceous (photo by Ashley B. Bennett, New Mexico State University).
Figure 27. Immature lady beetles are predaceous, while the adult stage feeds on pollen. Size varies from approximately 1–8 mm (1/25–3/10 inch) (photo by Miranda L. Kersten, New Mexico State University).
Figure 28. Tachinid flies are parasitoids that primarily attack the larval stage of their insect hosts (photo by Miranda L. Kersten, New Mexico State University).
Figure 29A. Adult syrphid flies visit flowers for nectar (photo by Ashley B. Bennett, New Mexico State University).
Figure 29B. The syrphid fly immature stage is predaceous (photo by Ashley B. Bennett, New Mexico State University).
Figure 30A. Lacewing adults can be identified by their long, lacey wings (photo by Ashley B. Bennett, New Mexico State University).
Figure 30B. The immature (pictured here) and adult stages of lacewings are predaceous (photo by Miranda L. Kersten, New Mexico State University).
Figure 31. The immature and adult stages of the minute pirate bug are predaceous. Minute pirate bugs are very small, with lengths of 2–3 mm (2/25–3/25 inch) (photo by Ashley B. Bennett, New Mexico State University).
Glossary
Augmentation biological control: Releasing insect predators and parasitoids to increase the natural enemy community.
Biological control: Using natural enemies to control pests.
Bt: Bacillus thuringiensis, a species of bacteria commonly used as a biological pesticide.
Cambium: A very thin layer of growing tissue just under the tree bark that produces new cells (phloem, xylem, or more cambium) and makes a tree’s trunk, branches, and roots grow larger in diameter. Several borers are known to mine this tender tissue.
Classical biological control: A method to control introduced pests by introducing natural enemies from the pest’s home range. These natural enemies are specialist predators or parasitoids of the introduced pest.
Complete metamorphosis: The developmental process during which an insect undergoes four stages: egg, larva, pupa, and adult. The adult stage lays the eggs that then hatch into larvae. Larvae feed for a time and then pupate. During pupation, the larvae change into the adult form, which emerge from the pupa. Each stage is visually different from the others. Larvae and adults often feed on different food sources, and sometimes larvae are the only stage that feed.
Conservation biological control: Manipulating the habitat to favor existing natural enemy populations; for example, by incorporating floral resources into the landscape.
Cornicle: A tube-like structure on aphids that projects from the abdomen (Figure 32).
Figure 32. A cornicle on an aphid (photo by Ashley B. Bennett, New Mexico State University).
Crawlers: The mobile nymph stage of mealybugs and scale insects.
Defoliation: Loss of leaves from a tree.
Elytra: A hard protective covering (modified front wings) over the hind wings, such as on lady beetles.
Entomopathogenic: An organism that parasitizes insects.
Frass: Waste produced by an insect that may appear similar to sawdust.
Gall: Abnormal growths on a plant, often caused by immature insects.
Incomplete metamorphosis: The developmental process during which an insect undergoes three stages: egg, nymph, and adult. Nymphs hatch from eggs and appear similar to adults. Adults are larger than nymphs and sometimes have wings.
Instar: Growth stages of an insect larva or nymph that is separated by a molt.
Larva: The immature stage of an insect. The larval stage of moths and butterflies is a caterpillar, and is a grub in beetles.
Metamorphosis: The developmental process of living organisms; in insects, metamorphosis can be complete or incomplete.
Mummy: A parasitized aphid; they can be distinguished from unparasitized aphids by their gray color and swollen appearance.
Natural enemies: Organisms that reduce populations of another organism through predation and parasitism.
Nymph: An immature stage of an insect that resembles its adult form.
Ovipositor: An organ with which a female insect lays eggs.
Parasitoid: A parasitic insect that requires a host to develop and whose development results in the death of its host.
Phloem: Tissue that carries sugar and nutrients from the leaves to the rest of the plant.
Pitch tubes: Areas where tree sap accumulates and that can identify where a pine beetle has bored into the tree.
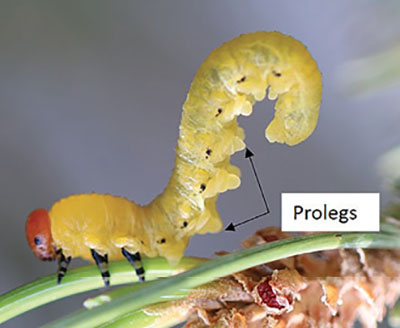
Prolegs: A foot-like appendage found on some insect larvae (Figure 33). Larvae may have legs and prolegs or only prolegs.
Figure 33. Prolegs are foot-like appendages found on some insect larvae (photo by Ashley B. Bennett, New Mexico State University).
Pubescence: Fine hairs on a leaf, often on the leaf underside.
Pupa: The developmental stage of insects in which the insect transitions from a larva to an adult; the pupal stage may be protected by a structure, such as a cocoon.
Skeletonization: Damage to the leaf caused by insects that feed between the veins and leave behind the leaf veins.
Solarization: The process of covering logs or soil with clear plastic so that the sun’s heat can kill pests.
Spumaline: A foamy substance that some insects cover their eggs with to protect them through the winter or from predators.
Stippling: Leaf damage that appears as small discolored spots, caused by feeding by sap-sucking insects.
Tubercles: Structures found on the pupal case of ash whiteflies that produce clear waxy droplets (Figure 34).
Figure 34. Tubercles on ash whitefly pupae produce clear waxy droplets (photo by Ashley B. Bennett, New Mexico State University).
Xylem: Tissue that provides support to a plant and moves water and dissolved nutrients from the roots upward to other parts of the plant.
Resources
Organic options and beneficial insects can be ordered online or from your local plant nurseries. Online sources include Arbico Organics (www.arbico-organics.com), Plant Natural Garden Supply (www.planetnatural.com), and Beneficial Insectary, Inc. (www.insectary.com).
References
Appleby, J.E., and R.B. Neiswander. 1962. Biology and control of the hackberry nipple and blister gall makers [Research Circular 111]. Wooster: Ohio Agricultural Experiment Station.
Bauer, L.S., and H.S. Pankratz. 1993. Nosema scripta N. sp. (Microsporidia: Nosematidae), a microsporidian parasite of the cottonwood leaf beetle, Chrysomela scripta (Coleoptera: Chrysomelidae). Journal of Eukaryotic Microbiology, 40, 135–141.
Cain, R., and D. Parker. 1998. Conifer pests in New Mexico. Albuquerque, NM: USDA Forest Service, Southwestern Region.
Ciesla, W.M., and I.R. Ragenovich. 2008. Western tent caterpillar [Forest Insect and Disease Leaflet 119]. Washington, D.C.: USDA Forest Service.
Ciesla, W.M., and D.R. Smith. 2011. Diprionid sawflies on lodgepole and ponderosa pines [Forest Insect and Disease Leaflet 179]. Washington, D.C.: USDA Forest Service.
Cranshaw, W. 1998. Pests of the West, 2nd ed. Golden, CO: Fulcrum Publishing.
Cranshaw, W., and D. Leatherman (Eds.). 2004. Insects and diseases of woody plants of the Central Rockies [Bulletin 506A]. Fort Collins: Colorado State University Cooperative Extension.
Dreistadt, S.H. 2004. Pests of landscape trees and shrubs: An integrated pest management guide, 2nd ed. [Publication 3359]. Davis: University of California Agricultural and Natural Resources.
Dreistadt, S.H., and A.B. Lawson. 2014. Pest notes: Elm leaf beetle [Publication 7403]. Davis: University of California Agriculture and Natural Resources.
Ellis, J.A., A.D. Walker, J.F. Tooker, M.D. Ginzel, P.F. Reagal, E.S. Lacey, A.B. Bennett, E.M. Grossman, and L.M. Hanks. 2005. Conservation biological control in urban landscapes: Manipulating parasitoids of bagworm (Lepidoptera: Psychidae) with flowering forbs. Biological Control, 34, 99–107.
Fairweather, M.L., J. McMillin, T. Rogers, D. Conklin, and B. Fitzgibbon. 2006. Field guide to insects and diseases of Arizona and New Mexico forests [MR-R3-16-3]. Albuquerque, NM: USDA Forest Service, Southwestern Region.
Galford, J.R. 1984. The locust borer [Forest Insect and Disease Leaflet 71]. Washington, D.C.: USDA Forest Service.
Godfrey, L.D. 2011. Pest notes: Spider mites [Publication 7405]. Davis: University of California Agriculture and Natural Resources.
Johnson, W.T., and H.H. Lyons. 1991. Insects that feed on trees and shrubs. Ithaca, NY: Comstock Publishing Associates.
Krischik, V., and J. Davidson. 2004. IPM (integrated pest management) of Midwest landscapes. St. Paul: University of Minnesota Agricultural Experiment Station.
McMillin, J.D., and M.R. Wagner. 1997. Chronic defoliation impacts pine sawfly (Hymenoptera: Diprionidae) performance and host plant quality. OIKOS, 79, 357–362.
Nguyen, R., and A.B. Hamon. 2000. Featured creatures: Ash whitefly [DPI Entomology Circular 337]. Gainesville: University of Florida.
Schowalter, T.D., and D.R. Ring. 2017. Biology and management of the fall webworm, Hyphantria cunea (Lepidoptera: Erebidae). Journal of Integrated Pest Management, 8, 1–6. https://doi.org/10.1093/jipm/pmw019
Seybold, S.J., T.D. Pain, and S.H. Dreistadt. 2008. Bark beetles: Integrated pest management for home gardeners and landscape professionals [Publication 7421]. Davis: University of California Agriculture and Natural Resources.
Smith, R.G., C.R. Ward, and L.M. English. 1998. Managing cottonwood leaf beetle [Circular 552; online]. Las Cruces: New Mexico State University Cooperative Extension Service. https://pubs.nmsu.edu/_circulars/circ552.html
Sutherland, C.A. 2006. Ornamental and turf guides [Online]. Las Cruces: New Mexico State University Cooperative Extension Service. http://www.nmda.nmsu.edu/wp-content/uploads/2016/09/Ornamental-Insects.pdf
USDA–ARS. 2018. A national road map for integrated pest management [Online]. https://www.usda.gov/oce/opmp/IPM%20Road%20Map%20FINAL.pdf
For Further Reading
H-169: Using Insectary Plants to Attract and Sustain Beneficial Insects for Biological Pest Control
https://pubs.nmsu.edu/_h/H169/
H-172: Backyard Beneficial Insects in New Mexico
https://pubs.nmsu.edu/_h/H172/
H-429: Trees of Young Park, Las Cruces, New Mexico
https://pubs.nmsu.edu/_h/H429/
Miranda Kersten is a Senior Program Specialist with the urban integrated pest management (IPM) program at NMSU’s Agricultural Science Center in Los Lunas. Her work focuses on pollinator and beneficial insect conservation, monitoring beneficial insects across urban landscapes, and managing IPM research projects.
This work is supported by the Community Forestry Assistance program through the New Mexico State Forestry Division of the New Mexico Energy, Minerals, and Natural Resources Department and USDA Forest Service.
In accordance with federal law and U.S. Department of Agriculture policy, this institution is prohibited from discriminating on the basis of race, color, national origin, sex, age, or disability. To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue SW, Washington, D.C. 20250-9410, or call (202) 720-5964 (voice and TDD). USDA is an equal opportunity provider and employer.
The pesticide recommendations in this publication are provided only as a guide. The authors and New Mexico State University assume no liability resulting from their use. Please be aware that pesticide labels and registration can change at any time; by law, it is the applicator’s responsibility to use pesticides ONLY according to the directions on the current label. Use pesticides selectively and carefully and follow recommended procedures for the safe storage and disposal of surplus pesticides and containers.
Brand names appearing in publications are for product identification purposes only. No endorsement is intended, nor is criticism implied of similar products not mentioned. Persons using such products assume responsibility for their use in accordance with current label directions of the manufacturer.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
December 2019, Las Cruces, NM