Circular 677
Revised by Rossana Sallenave
College of Agricultural, Consumer and Environmental Sciences
Author: Extension Aquatic Ecology Specialist, Department of Extension Animal Sciences and Natural Resources, New Mexico State University. (Print friendly PDF)
Introduction
The practice of using living organisms to measure the condition of the environment has been around for many years. Different measures of aquatic health—or indices—have become established scientific procedures that have been used for decades to monitor pollution levels of water bodies worldwide. In addition to being used by state and federal water resource agencies to assess the biotic integrity of water bodies, they have become an excellent educational tool used by students and citizen scientists for stream volunteer monitoring programs throughout the United States. The following guide is an introduction to freshwater biomonitoring aimed at students, hobby naturalists, anglers, or anyone interested in becoming involved in citizen monitoring programs to help assess and protect the health and integrity of our waterways.
The list of aquatic invertebrates described in the STREAM INVERTEBRATE IDENTIFICATION SHEET comprises groups of invertebrates typically found in and associated with waters of varying degrees of quality, from very good to poor. The tolerances refer to tolerances to organic pollution.
What is Biomonitoring?
Biological monitoring, or biomonitoring, is the use of living organisms or their responses to determine the quality of the environment they inhabit. It uses the presence or absence of indicator species or indicator communities to reflect environmental conditions. Stream biomonitoring is a method to evaluate the condition of a stream or river using biological surveys of the living organisms that inhabit the waters (Figure 1). It is a way of inferring the water quality based on what organisms are present. Unlike chemical measurements, which are like taking a single snapshot of the aquatic ecosystem, biological measurements provide a more long-term, integrative view of the water quality as reflected by the community of organisms inhabiting the aquatic environment. Benthic macroinvertebrates, fish, and/or algae can be used for biomonitoring. Macroinvertebrates are most frequently used and have the longest history of use in biomonitoring programs. Many state water quality agencies and citizen monitoring groups collect macroinvertebrate data. The Environmental Protection Agency (EPA) has developed and established protocols for using benthic macroinvertebrates to assess the water quality of surface waters.

Figure 1. Stream biomonitoring assesses the condition of a stream or river by surveying the living organisms found in the waters.
What are Benthic Macroinvertebrates?
Benthic (bottom-dwelling) macro (large enough to see with the naked eye) invertebrates (no backbone) are aquatic organisms that are generally small (will be retained by a 200- to 500-µm [0.01- to 0.02-inch] mesh) but large enough to be easily collected. These organisms inhabit bottom substrates of streams and lakes for at least part of their life cycles and inhabit all types of aquatic habitats. Some invertebrates are insects, but others include worms, crayfish, snails, and freshwater clams. Macroinvertebrates are the primary food source for many species of fish; therefore, environmental impacts on macroinvertebrates will have an effect on the food web and on the aquatic resources as a whole.
What are the Advantages of using Benthic Macroinvertebrates for Biomonitoring?
- They can be found in most aquatic habitats.
- They are affected by the physical and chemical conditions of the stream.
- They generally have limited mobility, so they cannot escape pollution events.
- There are a large number of different macroinvertebrate species. Some are more or less tolerant of pollution, and the presence or absence of certain species or groups of species reflects environmental conditions, which indicates good or poor water quality.
- Since benthic macroinvertebrates retain (bioaccumulate) toxic substances, chemical analysis will detect toxins in them when levels may be undetectable in the water resource.
- Small order streams often do not support fish, but do support extensive macroinvertebrate communities.
- They are small enough to be easily collected and identified.
- Sampling of macroinvertebrates is easy, requires few people and minimal equipment (e.g., nets, buckets, trays), and does not adversely affect other organisms.
What are the Disadvantages of using Macroinvertebrates to Infer Stream Water Quality?
- They do not respond to all types of pollutants.
- The presence or absence of a species may be due to factors other than pollution, such as unfavorable water currents, type of substrate, or drought.
- Their abundance and distribution may vary seasonally; therefore, comparisons of samples taken in different seasons may not be possible.
- Certain groups are difficult to identify to the species level.
There are two types of stream monitoring surveys:
- Surveys taken before and after a pollution event or before and after a project is complete to determine what, if any, effects the pollution event or project had on the overall water quality of the stream.
- Sampling at regular intervals throughout a specific time period. These surveys are done to monitor water quality over time to ensure that water quality is maintained and is in compliance with water use criteria.
- Qualitative surveys, which give a general assessment of the organisms present.
- Quantitative surveys, which will give an actual count of the number of different types of invertebrates present over a given area. This type of survey can be used in more advanced studies where statistical analyses can be conducted on the data collected. With these surveys you know the number of individuals per sample (abundance), how many species are present in the stream (“species richness”), and how many individuals of each species are present (“dominance”). Quantitative surveys can generate macroinvertebrate data for calculating biotic indices, which provide a better overview of the ecological condition, or biotic integrity, of your aquatic ecosystem.
What are Biotic Indices?
Biotic indices are used to classify the degree of pollution in an aquatic system based on the tolerance or sensitivity of different groups of invertebrates to pollution. While tolerance is generally not specific to the type of pollutant or stressor, the biotic index is oriented toward the detection of organic pollution. Organic pollution occurs when large quantities of organic compounds are released into watercourses. Organic pollutants originate from domestic sewage (raw or treated), urban runoff, industrial effluents, and farm waste. As microorganisms decompose these compounds, the dissolved oxygen in the receiving water may be used up, causing oxygen depletion and severe consequences for the stream biota.
The pollution tolerances of the different groups of macroinvertebrates included in the biotic index are based upon their tolerance to dissolved oxygen concentrations in the water. Organisms are separated into four categories of pollution tolerance: sensitive, semi-sensitive, semi-tolerant, and tolerant (corresponding to Groups 1 through 4 in the STREAM INVERTEBRATE IDENTIFICATION SHEET section). Indicator organisms in a sample are assigned scores according to their tolerance to the pollutant. The scores of the different sampled groups are summed up, and this value is used to identify the pollution status of the site. Stream health ratings are assigned based on the biotic index values and range from poor to good.
How Does Organic Pollution Affect Macroinvertebrates?
In streams with good water quality, you would generally expect to find a greater diversity of macroinvertebrate groups, which would include groups that are both sensitive and tolerant to pollution. As organic pollution increases, dissolved oxygen levels within the stream will fluctuate more extremely, and fewer organisms that are pollution-sensitive will be found. As organic pollution continues to increase, you would expect pollution-tolerant macroinvertebrates to become more dominant, while other sensitive and semi-sensitive organisms will not be able to survive.
Can Macroinvertebrates be used to Detect Other Types of Pollution besides Organic Pollution?
Freshwater benthic macroinvertebrates have frequently been used to monitor metals and organic contaminants such as pesticides and polychlorinated biphenyls (PCBs). It has long been known that organisms concentrate or accumulate pollutants in their tissues from their surrounding water. Animals such as mussels and crayfish are ideal sentinel organisms to monitor aquatic pollution because they are large, relatively long-lived, sedentary, easy to collect, and can often withstand high levels of the pollutant without being killed. Measuring metal and contaminants in water and sediment does not necessarily tell you how much of these compounds is bioavailable (can be absorbed into a living system) to organisms living in the water. By measuring the levels of these contaminants in the tissues of sentinel organisms, you know how much of these pollutants were taken up by living organisms. Macroinvertebrates have been used in other ways to assess the impacts of heavy metals in aquatic systems. In addition to using sentinel species to measure bioaccumulation of metals and other compounds, a number of studies have measured changes in benthic invertebrate community composition in response to heavy metals in streams.
Where Can I Learn More About using Macroinvertebrates Biotic Indices (MBI)?
The following sites contain more information on identifying macroinvertebrates and on calculating MBIs to assess water quality:
Macroinvertebrate Biotic Index Calculator—converts your collection data into quantifiable pollution levels— http://peterchen.nicerweb.com/RiverWatch/MBI_calculator.html
Wave Action Volunteers Biotic Index— https://wateractionvolunteers.org/files/2020/01/WisStreamCurriculum-Section4.pdf
Texas Identification Key for Colorado River—https://austintexas.gov/sites/default/files/files/Watershed/hydrofiles/macro_key.pdf
Key to macroinvertebrates in Utah—https://extension.usu.edu/waterquality/htm/macrokey
Stream Invertebrate Identification Sheet
Group 1: These are sensitive to organic pollutants and require high dissolved oxygen levels
Stonefly nymphs
(Order Plecoptera; Figures 2–4)
Description: 1–4 cm (0.4–1.6 inches) long; six legs, each ending in short, paired claws; visible antennae; two elongated tails or cerci (never three); no gills on abdomen.
Feeding: Most species feed on decaying plants or animals, but some feed on bacteria, while others (Family Perlidae) are predators.
Habitat: Swiftly moving streams with high oxygen levels.

Figure 2. Stonefly nymph (Family Perlidae) (photo courtesy of Society of Freshwater Science [SFS; formerly North American Benthological Society or NABS]).

Figure 3. Stonefly nymph (Family Perlodidae) (photo courtesy of SFS).

Figure 4. Stonefly nymph (Family Pteronarcyidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Dobsonfly larvae
(Order Megaloptera; Figure 5)
Description: Up to 10 cm (4 inches) long when fully grown; six legs; large, aggressive jaws; 1–8 lateral filaments on abdominal segments, abdomen ends in a pair of filaments, each with two hooks.
Feeding: Predators with powerful chewing mouthparts.
Habitat: High-quality streams; usually lives under rocks in running fresh water.

Figure 5. Dobsonfly larva (Family Corydalidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Alderfly larvae
(Order Megaloptera; Figures 6 and 7)
Description: Up to 2.5 cm (1 inch) long; six legs, with 1–7 lateral filaments on abdominal sections; the last abdominal section terminates in one single filament (caudal filament).
Feeding: Aggressive predators.
Habitat: High- or medium-quality water. Alderfly larvae are more tolerant of pollution than dobsonflies and may be found in areas impacted by pollution.

Figure 6. Alderfly larva (Family Sialidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
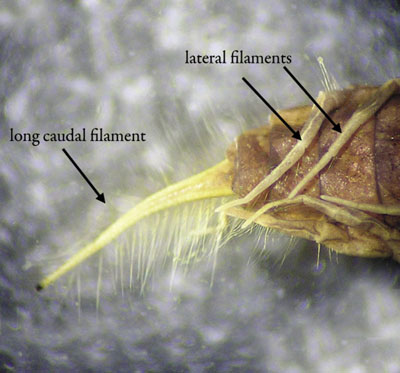
Figure 7. Abdomen of alderfly larva showing lateral filaments and tail projection (caudal filament) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Watersnipe fly larvae
(Order Diptera, Family Athericidae; Figure 8)
Description: Up to 3 cm (1.2 inches) long; cylindrical and slightly flattened body; cone-shaped abdomen; many legs with suction tips; posterior end has a pair of fringed filaments that look like antennae; pale to green in color.
Feeding: Predators.
Habitat: Inhabits very clean, flowing waters.

Figure 8. Watersnipe larva (Family Athericidae) (photo courtesy of SFS).
Group 2: These are semi-sensitive to pollutants and require moderate to high dissolved oxygen levels.
Mayfly nymphs
(Order Ephemeroptera; Figures 9–11)
Description: 0.3–3 cm (0.1–1.2 inches) long; six legs, each ending in a single claw; visible antennae; three long tails (sometimes two); plate-like or feathery gills along sides of abdomen.
Feeding: Grazers or gatherers that feed on algae and organic debris.
Habitat: Some cling to rocks, some burrow in the mud, and others are free swimmers. Some species in the family Baetidae are more tolerant of pollutants than others. Diversity of mayfly species decreases with stream degradation.

Figure 9. Mayfly nymph (Family Caenidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).

Figure 10. Mayfly nymph (Family Baetidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).

Figure 11. Mayfly nymph (Family Heptageniidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Caddisfly larvae
(Order Trichoptera; Figures 12–14)
Description: Up to 2.5 cm (1 inch) long; six legs, each with hooked claws; two small hooks at the end of the abdomen; some species build cases of small stones, sticks, or leaves, while others are free-living or spin nets that attach to rocks.
Feeding: Some graze algae, others filter-feed on organic waste or debris, and a few free-living species are predators.
Habitat: High-quality streams; some (such as Hydropsychidae) are tolerant of mild pollution.

Figure 12. Case-building caddisfly (Family Limnephilidae) (photo courtesy of SFS).

Figure 13. Net-spinning caddisfly (Family Hydropsychidae) (photo courtesy of SFS).

Figure 14. Caddisfly larva (Family Lepidostomatidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Beetle larvae and adults (Order Coleoptera)
Water penny larvae (Family Psephenidae; Figures 15 and 16)
Description: 0.3–0.7 cm (0.1–0.3 inch) long; broad, flat, saucer-shaped body; six legs underneath.
Feeding: Grazes algae and other material attached to rocks.
Habitat: Clings to undersides of rocks and other debris in cold, fast-running, high-quality streams.
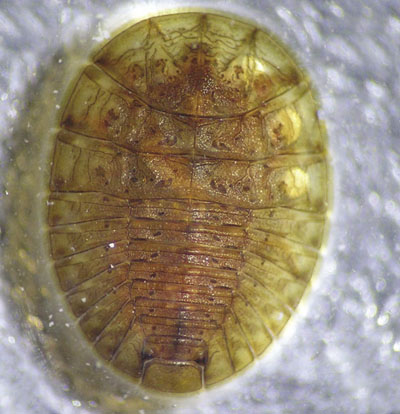
Figure 15. Water penny larva (Family Psephenidae) top (dorsal) view (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
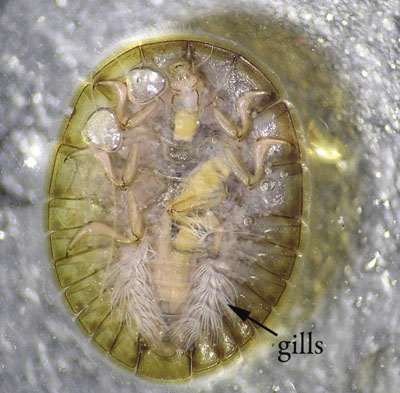
Figure 16. Water penny larva bottom (ventral) view (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Riffle beetle larvae and adults
(Family Elmidae; Figures 17 and 18)
Description: Larvae are 3–16 mm (0.1–0.6 inch) long; stiff, cylindrical body; six legs; small tuft of white filaments at tail end. Adults are 2–8 mm (0.08–0.3 inch) long; black; look similar to many terrestrial beetles; their legs are long and have two claws.
Feeding: Collects and gathers algae, diatoms, and organic debris.
Habitat: Larvae of many species cling to rocks in stream riffles. Adults walk slowly along stream bottoms in stream riffles.
Note: Unique in that both larval and adult stages are aquatic.

Figure 17. Riffle beetle (Family Elmidae) larva (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).

Figure 18. Riffle beetle (Family Elmidae) adult (photo courtesy of SFS).
Dragonfly and damselfly nymphs(Order Odonata)
Dragonfly nymphs (Suborder Anisoptera; Figure 19)
Description: 1.25–5 cm (0.5–2 inches) long; large protruding eyes; round to oval abdomen; six legs, each with short, paired claws.
Feeding: Predators that feed on aquatic insects, tadpoles, and small fish.
Habitat: Slowly moving water (ponds or pools). Able to withstand low oxygen levels and are thus more tolerant of organic matter pollution.

Figure 19. Dragonfly nymph (Family Libellulidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Damselfly nymphs
(Suborder Zygoptera; Figure 20)
Description: 0.25–5 cm (0.1–2 inches) long; large protruding eyes; six thin legs; long, thin abdomen ending with three leaf-like gills.
Feeding: Predators that feed on small aquatic invertebrates.
Habitat: Typically found in medium-quality, slowly moving water (pools and ponds). Able to withstand low oxygen levels.

Figure 20. Damselfly larva (Family Coenagrionidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Cranefly larvae
(Order Diptera, Family Tipulidae; Figure 21)
Description: Usually 1–2.5 cm (0.4–1 inch) long, but can be as long as 10 cm (4 inches); large, fleshy, segmented, and worm-like, resembling small cigars; four fingerlike gill lobes at the end of the abdomen; light brown, green, or milky in color.
Feeding: Most species graze on algae or are gatherers feeding on decaying organic matter, but a few are predators.
Habitat: Can be found in moss, silt, and leaves at the bottom of either standing or flowing waters.

Figure 21. Cranefly (Family Tipulidae) larva (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Crayfish
(Order Decapoda; Figures 22 and 23)
Description: Up to 15 cm (6 inches) long; resemble small lobsters, with two large claws and eight smaller, jointed walking legs.
Feeding: Considered omnivores. Eats fish, aquatic vegetation and invertebrates, plankton, as well as dead plants and animals. Uses large claws to tear plant and animal prey into small chunks.
Habitat: Slow-moving streams, rivers, and ponds. Crayfish require waters with adequate amounts of oxygen, but can tolerate lower oxygen levels than other species in Group 2. Crayfish are included in Group 2 because they are sensitive to toxic substances that can accumulate in plants or animals they may eat.

Figure 22. Adult female crayfish (Family Cambaridae) (photo courtesy of SFS).

Figure 23. Adult crayfish (Family Cambaridae) (photo courtesy of SFS).
Group 3: These are semi-tolerant of pollutants and can survive with moderate oxygen levels.
Blackfly larvae
(Order Diptera, Family Simuliidae; Figure 24)
Description: Up to 0.5 cm (0.2 inch) long; head has feathery gills; end of abdomen has a suction pad; overall body shape resembles a bowling pin.
Feeding: Filters small particles of organic matter in water currents.
Habitat: Lives attached to submerged rocks; requires swiftly flowing water.

Figure 24. Blackfly (Family Simuliidae) larva (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Non-red midge larvae
(Order Diptera, Family Chironomidae; Figure 25)
Description: Up to 1.25 cm (0.5 inch) long; have a worm-like body and distinct head; one or two leg-like “prolegs” just behind the head capsule; body often C-shaped; off-white in color.
Feeding: Feeds on a variety of organic substances (detritus, algae, fungi, and in some cases other midge larvae).
Habitat: Midges are found in all water types, including salt water. They live in the silt bottom, on solid substrates, or on aquatic plants. Tolerant of some pollution. High numbers of some types (see Group 4) indicate poor water quality, particularly in the absence of other species.
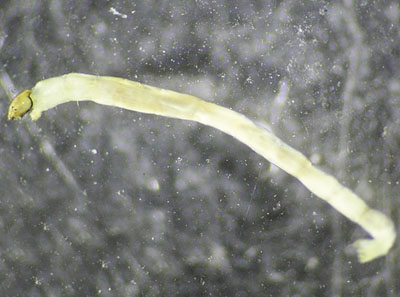
Figure 25. Midge larva (Family Chironomidae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Scuds
(Order Amphipoda; Figure 26)
Description: Up to 1.25 cm (0.5 inch) long; swim rapidly on their sides and resemble shrimp; laterally compressed, hump-shaped back; several pairs of legs; white, grey, or pink in color.
Feeding: Gathers dead and decaying matter.
Habitat: Some are highly sensitive to pollution; others can be found in moderately polluted water.

Figure 26. Amphipod (Family Gammaridae) (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Group 4: These are tolerant of pollutants and can live in streams with low dissolved oxygen.
Tubificid worms
(Class Oligochaeta, Family Tubificidae; Figure 27)
Description: Long (usually less than 7.5 cm [3 inches]), thin, segmented worms with no legs.
Feeding: Ingests mud and filters out organic material.
Habitat: Found in all types of water, including highly polluted areas. Worms of the genus Tubifex are tolerant of pollution and can be found in severely polluted waters; high numbers indicate poor water quality.
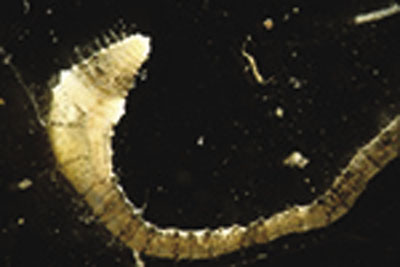
Figure 27. Aquatic worm (Family Tubificidae) (photo courtesy of SFS).
Bloodworm midges (red)
(Order Diptera, Family Chironomidae; Figure 28)
Description: Up to 1.25 cm (0.5 inch) long; have a worm-like body and distinct head; two leg-like “prolegs” right below the head capsule; bright red in color, referred to as “blood worms.”
Feeding: Feeds on a variety of organic substances (detritus, algae, fungi, and in some cases other midge larvae).
Habitat: Lives in the silt bottom, on solid substrates, or on aquatic plants. Can be found in severely polluted waters; high numbers indicate poor water quality.

Figure 28. Bloodworm midge (third from left) (Family Chironomidae) (photo courtesy of SFS).
Leeches
(Order Hirudinea; Figure 29)
Description: Up to 5–8 cm (2–3 inches) long; segmented but with wrinkled integument (outer covering); bodies worm-like and stretchable, slimy, flattened, with sucker at each end; tan or brown in color.
Feeding: Some species are scavengers, feeding on dead animal matter; more are carnivorous, feeding on snails, insects, worms, and other invertebrates. Some species occasionally or regularly (depending on the genus) suck blood from fish, frogs, and humans.
Habitat: Indicators of low dissolved oxygen. Can be found in severely polluted waters.

Figure 29. Leech (Family Glossiphoniidae) adult (photo courtesy of SFS).
Pouch or bladder snails
(Class Gastropoda; Figure 30)
Description: 0.5–2 cm (0.2–0.8 inch) long; cone-shaped shell surrounding a soft, muscular body.
Feeding: Scrapes algae and bacteria from submerged surfaces.
Habitat: These snails do not have primary gills, but instead obtain oxygen through a “lung” or pulmonary cavity. They can live in waters with little oxygen and can be found in severely polluted waters.
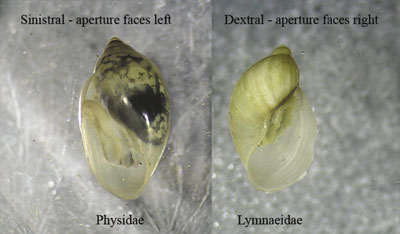
Figure 30. Bladder snails (left; Family Physidae) are air-breathing snails that are widespread and can tolerate water with little or no oxygen. They can be distinguished from other snails (such as Lymnaeidae snails shown on right) by their sinistral shell, which means that if the shell is held so the spire (the tapering point of the shell) is pointing up and the aperture (shell opening) is facing the observer, the aperture is on the left-hand side (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Aquatic sowbugs
(Class Crustacea, Order Isopoda; Figure 31)
Description: 0.5–2 cm (0.2–0.8 inch) long; relatively flat; have long antennae and seven pairs of legs.
Feeding: Feeds on both dead and live plants and animals.
Habitat: Can tolerate high levels of decaying organic matter and very low oxygen levels; typically found in muddy, slow-moving waters. Can be found in fairly polluted waters.

Figure 31. Isopod adult (photo courtesy of the California Department of Fish & Game Aquatic Bioassessment Laboratory).
Additional Sources of Information on Biomonitoring, Techniques for Macroinvertebrate Sampling, and Examples of Studies Using Macroinvertebrates to Study Impacts of Heavy Metals on Streams
Webpages
EPA Biological Assessment of Wetlands—https://water.epa.gov/type/wetlands/assessment/bawwg_index.cfm
EPA Monitoring Water Quality—Biological assessment—https://water.epa.gov/type/watersheds/monitoring/bioassess.cfm
Documents
Barbour, M.T., J. Gerritsen, B.D. Snyder, and J.B. Stribling. 1999. Rapid bioassessment protocols for use in streams and rivers: Periphyton, benthic macroinvertebrates and fish, 2nd ed. [EPA 841-B-99-002]. Washington, D.C.: U.S. Environmental Protection Agency, Office of Water.
Clements, W.H. 1994. Benthic invertebrate community responses to heavy metals in the Upper Arkansas River Basin, Colorado. Journal of the North American Benthological Society, 13, 30–44.
Clements, W.H., D.S. Cherry, and J.H. Van Hassel. 1992. Assessment of the impact of heavy metals on benthic communities at the Clinch River (Virginia): Evaluation of an index of metal impact. Canadian Journal of Aquatic Sciences, 49, 1686–1694.
Rosenberg, D.M., and V.H. Resh (Eds.). 1993. Freshwater biomonitoring and benthic macroinvertebrates. New York: Chapman & Hall.
For further reading
W-103: Managing Filamentous Algae in Ponds
https://pubs.nmsu.edu/_w/w-103/
W-104: Understanding Water Quality Parameters to Better Manage Your Pond
https://pubs.nmsu.edu/_w/W104/
W-105: Understanding and Preventing Fish Kills in Your Pond
https://pubs.nmsu.edu/_w/W105/index.html
CR-647: Toxic Golden Algae (Prymnesium parvum)
https://pubs.nmsu.edu/_circulars/cr-647/
CR-681: Managing Aquatic Weeds
https://pubs.nmsu.edu/_circulars/CR681/index.html

Rossana Sallenave is an Extension Aquatic Ecology Specialist at New Mexico State University. She earned her Ph.D. at the University of Guelph in Canada. Her research interests include aquatic ecology and ecotoxicology. Her Extension goals are to educate and assist New Mexicans on issues relating to watershed stewardship and aquatic ecosystem health.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
Revised February 2023


