Circular 629
John J. Ellington, Tracey Carrillo, Jill McCauley, Denise McWilliams, Jay Lillywhite, Jane Pierce, Jeff Drake
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Authors: Respectively, Professor (joelling@nmsu.edu); Senior Research Specialist; Research Assistant, all of the Department of Entomology, Plant Pathology and Weed Science; former Extension Specialist, Department of Extension Plant Sciences; Assistant Professor, Department of Agricultural Economics, all of New Mexico State University; Assistant Professor, Department of Entomology, Plant Pathology and Weed Science, New Mexico State University, Artesia; and Adjunct Assistant Professor, USDA APHIS researcher, New Mexico State University. (Print Friendly PDF)
Abstract
Although yields of cotton can be increased with higher rates of nitrogen use, the highest crop yields do not necessarily give the highest profits. Precision tracking of plant stress and insect infestations in New Mexico cotton fields showed that increased cotton growth under a higher-N regime is offset by increases in populations of pest insects (e.g., Lygus spp). For this project a new high clearance research vehicle with precision infrared plant stress sensors (GreenSeeker™), precision granular/liquid fertilizer applicators and a high vacuum insect collector was designed and assembled in early 2006. Yield and nitrogen levels were found to correlate highly (R2 = 0.93 in 2004, 0.83 in 2005) with Normalized Difference Vegetation Index (NDVI) readings, which indicate plant stress. There was a nitrogen–Lygus spp. adult density interaction. Overall densities of Lygus spp. adults were reduced ~50% in insecticide-free years (2005 and 2006), while profits were increased, when nitrogen use rates were reduced from ~135 to 80 lbs N/acre. Predator densities were independent of nitrogen use rates. Five irrigations and 80 lbs of nitrogen/acre optimized profit on Harkey clay loam soils in New Mexico. Fertilizers and pesticides have a high economic cost for producers as well as a negative effect on the environment, and higher rates of use do not increase profit margins for cotton producers in New Mexico. Future insect control practices should be based on analysis of profit rather than yield. Precision/conservation farming methods (using NDVI readings and improved insect sampling) result in lower use of nitrogen fertilizer, water, insecticides, plant hormones and defoliants, thus sustaining natural predator/prey ratios, reducing expenditures of time and money and improving profit margins.
Acknowledgements
This project was partially funded by WERC, a consortium for environmental education and technology developments.
Materials and Methods and Validation of Results
Introduction
Nitrogen ultimately limits nearly all plant and animal growth on earth, being required for the development of RNA, DNA, amino acids and proteins (White, 1993). High-yield technology based on the increased use of nitrogen and pesticides, introduced during the green revolution, has helped farmers produce on average three times higher yields on their land (Stovig, 2004).
World nitrogen use between 1997–1998 and 1999–2000 was 120–137 million tons (International Fertilizer Industry Association, 2000). The nitrogen use rate in the United States during this period was between 11.5 and 12.5 million tons. Between 472,000 and 567,000 tons were applied to cotton (USDA Economic Research Service, 2006).
World expenditures for pesticides in 2000–2001 were $32 billion, of which $11 billion was spent in the United States. Insecticides accounted for $3.6 billion of the total U.S. pesticide expenditures (U.S. Environmental Protection Agency, 2006). If nitrogen and pesticide use in the U.S. could be reduced by even 10% it would save some 1.2 million tons of nitrogen and $3.2 billion in pesticide expenditures.
About half of this applied nitrogen is not taken up by plants but is lost to the atmosphere and to above- and below-ground water supplies (Smil, 1997). Herrera (2001) found 77% of nitrogen applied to pecans in New Mexico is lost to ground water and the atmosphere. In an effort to ensure optimum crop production, growers often use more nitrogen and water than is necessary. Warden et al. (1992) found that a 50% reduction in use of both nitrogen and water did not compromise yields in California lettuce production in the Salinas Valley and was the point of maximum profit.
Because nitrogen fertilizer has been inexpensive, and because precision measuring and application methods have not been sufficiently developed, growers throughout the world have generally tended to apply far more nitrogen than needed for crop production. This has led to a plethora of problems. The introduction of large quantities of nitrogen to soil, water and atmosphere is profoundly detrimental; its consequences include contamination of underground and aboveground water supplies—causing algal blooms—and contamination of the atmosphere (Simone, 2005). High nitrogen and insecticide use has been linked to many cancers and to other health issues including Parkinson's disease and allergies (Simone, 2005). Of special significance to this study, excessive nitrogen drives up population densities of plant and animal pests cf 100 (White, 1993), particularly in monocultures (Conway & Pretty, 1991).
Initial insecticide use, to control primary pests, kills both pest and beneficial insect populations and often leads to multiple applications over large land masses, which select out resistant biotypes that eventually may not be controllable. In contrast, phytophagous insect problems can often be reduced with proper cultural practices, that is, by avoiding over-consumption of nitrogen and water (Leigh, 1996; Altieri & Nicholls, 2003) and by providing refugia for beneficial insects (Garr et al., 2000).
New Mexico has been an ideal place to carry out this work because the primary exotic harmful insects in New Mexico (the boll weevil, Anthonomous grandis grandis Boheman, and the pink bollworm, Pectinophora gossypiella [Sanders]) have been reduced or completely eradicated, opening the way for the use of integrated biological control techniques. In cotton growing counties in New Mexico, numerous small alfalfa fields dispersed among the cotton fields (74,000 acres of cotton vs. 115,000 acres of alfalfa) provide a perfect model for management of complex integrated systems.
Cotton has been chosen as a model system because it is one of the most difficult crops to manage, it is one of the largest users of nitrogen and pesticides in the world, and it is supported by governmental subsides—if these subsidies are lost U.S. growers will be forced to optimize production practices to compete in world markets.
Background
Agronomic Factors
In most crops, water and nitrogen are the most limiting factors for plant growth. Farmers need to know the relationship between profit, water and nitrogen use, that is, the cost–benefit ratio. Factors such as water quality, evapotranspiration and soil type interact to influence water use. Similarly, efficient nitrogen application rates depend on timing, application methods, soil type, nitrogen sources and carryover.
Determining Water and Nitrogen Availability
Traditionally, water and nitrogen monitoring in complex agricultural production systems was difficult and labor intensive; however, both water and nitrogen can be monitored quickly and effectively by measuring plant stress. Plant stress measurements determine health irrespective of the factors that modify water and nitrogen availability as plant growth occurs.
Optimizing Plant NDVI Sampling
Raun et al. (2002, 1998) showed that significant differences in total available nitrogen, NH4-N and N03-N, exist in soil areas 3.2 ft2 or less in size. Therefore, precision applications of fertilizer and nitrogen could theoretically optimize profit while reducing nitrogen use. NTech Industries Inc. and Oklahoma State University jointly developed the GreenSeeker™, which measures plant stress as a function of biological differences which exist in the field. Active lighting optical sensors on the GreenSeeker™ use high-intensity light emitting diodes (LEDs) that emit 600 nm (red) and 780 nm (near infrared) light sources. These LEDs are pulsed at high frequencies. Magnetic filters remove all background illumination. Magnitude of the filtered signal is measured by a multiplexed A/D converter. The sensors are temperature stable. One hundred readings/12.9 ft2 are taken, averaged and plotted using GPS; from this, a normalized difference vegetative index (NDVI) is calculated (NDVI = IR - R/IR + R, where IR = infrared, R = red light). NDVI technology can be used to target places where site-specific fertilizer applications should be made. Measuring stress from GreenSeeker™ readings has the advantage over traditional soil sampling of being fast and reliable, and over aerial stress measurements of providing fast data turnaround without interference from cloud cover. Significant increases in nitrogen use efficiency of, on average, 15% have been reported in multiple wheat fields using GreenSeeker™ technology (Raun et al., 2002).
Insect Factors
Host Seeking by Insects
Insects find host fields passively, by being blown into them, or actively, by following a scent trail upwind to them. Insects within fields predominately rely on plant-produced, volatile, soluble, secondary compounds to locate optimal host plants. In the head-space of intact plants, generally 10–100 volatile compounds occur (Schoonhoven et al., 1998). The major groups of components released are six-carbon alcohols, aldehydes and esters of acetates and other short chain aliphatic acids, and a variety of variable range mono- and sesquiterpenoids (Van Loon & Dicke, 2001). In any plant species, the composition and quantity of volatiles emitted is prone to considerable variation (Dicke & Vet, 1999). The most important sources of variation are genotype, developmental stage and biotic factors such as competing plants, infections by pathogens and arthropod damage. Abiotic factors that affect production of volatiles are, for example, shading and soil nutrient level. Nitrogen levels may have profound effects on phytophagous insect densities, affecting not only amino acid and protein synthesis but also the synthesis of volatile compounds, which cue insects as to the most viable hosts (i.e., those with the highest nitrogen levels).
In reviewing 50 years of research relating to crop nitrogen and insect attack, Scriber (1984) found 135 studies showing increases in damage to plants or growth in populations of leaf-chewing insects or mites in nitrogen fertilized crops, versus fewer than 50 studies in which herbivore damage was reduced by normal fertilizer regimens. Of 100 studies on insects and mites in Letourneau's (1988) review, two thirds showed an increase in growth, survival, reproduction rate or population densities of insects and mites, or an increase in plant damage levels, in response to increased nitrogen fertilizer use. The concentration of nitrogen in food plants generally plays a dominant role in the nutritional ecology of insect species (Soldaat & Vrieling, 1992).
The influence of varying levels of soil fertility has been documented for certain cotton insects, including the cotton aphid, Aphis gossypii Glov. (McGarr, 1942; Isley, 1946), species of mirids (e.g., the cotton flea hopper, Psallus serialus [Reut.]; Butt et al., 1946; Adkisson, 1957), Lygus spp. (Leigh & Goodell, 1996), and the bollworm Heliocoverpa zea (Boddie) (Fletcher, 1929; Thomas & Dunnam, 1931). Gains (1932, 1933) reported that the close correlation between rate of oviposition by bollworm moths and rate of plant growth suggests rapidly growing cotton is a preferred site. Fletcher (1941) correlated the number of bollworm larva present in different fields with the moisture content of the growing tips of cotton plants. Swezey et al. (2004) found that compared to conventional fields organic fields had more generalist predators and significantly fewer nymphs of the western tarnished plant bug (Lygus hesperus Knight).
Sampling Insects
Because traditional hand-net samples catch only ~8% of the total insects present in cotton (Ellington et al., 1984a) and the beneficial complex is normally not counted, an attempt was made to improve speed, accuracy and inclusiveness of insect sampling.
Acquiring Samples—In 1983, a small (hydraulically driven) self-propelled platform (Insectavac) with a 4,200-cfm high-vacuum fan was designed and built to take representative cotton insect samples (Ellington et al., 1984b). In 2006, an identically performing research vehicle (RV) was constructed (Figure 1); in addition to the vacuum system it uses three GreenSeeker™ sensors mounted over cotton rows in front of the vehicle. These control solenoid release valves for liquid or granular fertilizer applications mounted in back of the vehicle. These samplers can cross borders and are easily transported from field to field.

Figure 1. 4WD vehicle with IR, GPS, variable rate fertilizer applicator and insect vacuuming system.
Calibration—The Insectavac collector was calibrated for 24 genera of insects by comparing eighty-three 100-ft relative vacuum samples in three cotton fields to eight hundred thirty 2.4-ft absolute clam shell samples in the same plots. Efficiency ranged from 14–64%, average 32.6%, depending on insect species, densities, clumping patterns and plant height. Insectavac sample densities can be converted to absolute or sweep net densities when needed (Ellington et al., 1984a).
Sample Size—The optimum size and number of replications needed to estimate the mean density of 24 genera of insects in cotton was determined by vacuum sampling 100-ft quadrates end to end 12 times over a three-year period in three cotton fields (590 samples). Data for each date and location were pooled and the data analyzed as a nested design (Figure 2). Variability in the data set increased with sample length because the samples went over clumped and nonclumped areas. In practice, the optimum sample size was found to be the smallest sample size possible while maintaining statistical integrity. In New Mexico, a good compromise is 100 row feet replicated four times in a given field. Four 100-ft vacuum samples per cotton field generally catch on average 32% of the total insects present, depending on plant height, density, and clumping patterns of species evaluated (Ellington & Southward, 1996; Ellington et al., 1988; Ellington et al., 1984a, b; Amin et al., 1994).
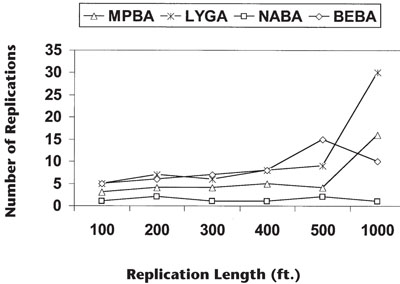
Figure 2. Number of replications needed to estimate the mean density of minute pirate bug adults (MPBA), lygus adults (LYGA), nabid adults (NABA), big eyed bug adults (BEBA) to within 80±5% of their true mean at various replication lengths.
Predicting Primary Consumer Densities
The complex group of arthropods found in agricultural ecosystems may be composed of host-specific and host-nonspecific primary consumer, parasitoid, predator and hyperparasitoid species. These arthropods may interact in positive, negative, or neutral ways, depending on behavior and factors that influence natality, mortality, speed of development and migration (Ellington et al., 1988). Each system must be evaluated on its own merits.
Predator Switching
Some parasitoid species and most predaceous arthropods are relatively host-nonspecific (Ellington et al., 1997). A host-switching parasitoid or predator can stabilize an otherwise unstable host–parasitoid interaction (Murdock, 1969).
Migration
Alfalfa remains a primary source of Lygus spp. and beneficial insects in many western agricultural regions. We have documented 120 species of parasitoids and two dozen predators which occur in cotton and alfalfa fields in New Mexico. These beneficial insects and Lygus spp. may migrate 1/4–1/2 mile to adjacent cropping systems each time an alfalfa field is cut (Ellington et al., 2001; Ellington et al., 2003; Loya, 2002). A similar system was documented at the Boswell farms, Corcoran, CA (Figure 3), when beneficial insects (lacewings [Chrysoperla spp.], minute pirate bugs [Orius spp.] and bigeyed bugs [Geocoris spp.]) and Lygus spp. were tracked downwind from a drying safflower field to cotton with an Insectavac sampler (Ellington et al., 2003). Because the safflower was no longer attractive these insects remained in the cotton.
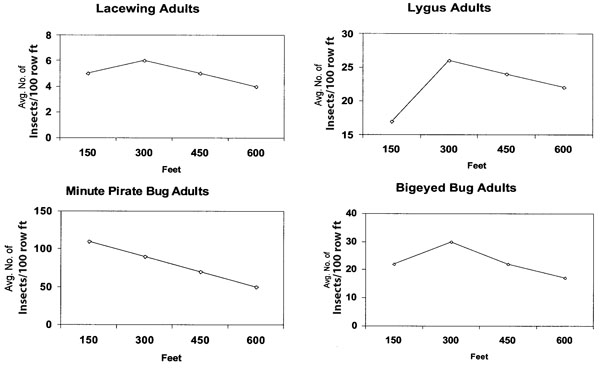
Figure 3. Average of four 100-ft Insectavac insect samples taken 150, 300, 450, 600 ft from the edge of a 360-acre cotton field downwind from a 360-acre drying safflower field, Boswell farms, Corcoran, CA.
Carriere et al. (2006) found that forage and seed alfalfa as well as weeds act as L. hesperus sources for cotton and that these sources did not extend beyond 1,230, 1,640 and 4,921 ft respectively. Conversely, cotton acted as a L. hesperus sink up to 2,460 ft.
Sevacherian and Stern (1975) found that Lygus spp. populations in cotton are often itinerant adults that move into cotton when nearby alfalfa hay fields are harvested. These adults usually leave the cotton and seek alfalfa fields with new regrowth on fields that have not been moved. Similar dispersal characteristics have also been noted by McGregor (1927), Smith (1942), Owen (1962), and Stern et al. (1964; 1967), and were the basis for the alfalfa interplant technique used in cotton (Stern et al., 1969).
Work by Stewart and Gaylor (1994) found that older L. hesperus females with chlorinated eggs are more likely than either males or females less than 8 days old to engage in the type of long-duration flight that results in migration into cotton. Based on results of sticky trap captures, approximately 90% of L. hesperus flight occurs within 6 ft of ground level (Stewart & Gaylor, 1991; Ridgway & Gyrisco, 1960). This tendency of L. hesperus to fly near the ground may be one reason that higher concentrations of L. hesperus often are observed near the periphery of cotton fields (Layton, 2000). These observations are consistent with our results on Lygus spp. migration in New Mexico (Figure 3).
In New Mexico, the density of L. hesperus and the beneficial complex in cotton returns to previous levels shortly after the residues from insecticide applications dissipate (Ozkock, 1997; Loya, 2002), suggesting constant migration of L. hesperus and the beneficial complex from recently cut alfalfa to standing alfalfa or to other cropping ecosystems such as cotton; however, as in other states, alfalfa appears to out-compete cotton for a variety of insects common to both crops.
Predator Feeding Preferences
Ellington et al. (1997) in an extensive six-year study based on over 1,200 (100-ft) samples taken with the Insectavac vacuum sampler in 31 different cotton fields found predators were associated with various primary consumers 163 times and various other predators 191 times, suggesting switching by predators may occur readily. If this is true, an invading primary consumer might be primarily controlled by nonhost-specific, switching predators. Predator-rich systems may have an element of stability not found in predator-poor systems.
Control of Primary Consumers by Predators
To determine the power of predators in controlling phytophagous insect species in fields, five bollworm eggs per plant were glued to 40 cotton plants (200 eggs) five times in three cotton fields in 1998 (10,000 eggs per field). Egg mortality was evaluated daily for two days after initiation of the test. Fifty percent and 85% bollworm egg mortality occurred one and two days, respectively, after initiation of the test. Final R2 values from regressions of total predator densities on bollworm egg mortality in these cotton fields were above 90%. Additional mortality might be expected from third day exposure of eggs and young larvae to predators, parasitoids and possibly weather factors (Ellington & El-Sokkari, 1986).
Lygus spp. Damage and Compensation
The impact of L. hesperus on cotton square yield loss is controversial because of conflicting experimental results and different interpretations of cotton's ability to compensate (Rosenheim et al., 2006). Rosenheim et al. (2006) found mobile transit populations of L. hesperus sweeping through fields in the San Joaquin Valley did not seem to explain higher- or lower-than-expected levels of square abscission, and Gutierrez et al. (1979) suggested L. hesperus is not a serious pest of cotton in the Central Valley of California in most years and that yield fluctuations were caused by weather patterns and not by insects.
Chapman (2002) and Kerby et al. (1987) reported Acala cottons, even under ideal conditions, drop 60% of young squares, and that defoliation up to 70% had no economic effect. Pierce et al. (in press) found that one-time mechanically applied injury, done by removing all 1/3 and younger squares to simulate a single event of insect damage mid and late August, did not compromise yield; however, repeating this procedure two times did significantly reduce yield. Wilson et al. (2003) suggested early season pest damage to cotton is largely cosmetic. Crop yield and crop maturity were not affected by 100% defoliation before first flower buds appeared, and 100% fruit removal from the first four fruiting branches did not affect yield but delayed maturity seven days. Cotton can compensate for large square losses. Compensation may be modified by stress, e.g., season length, cloudiness, water availability, nutrient availability, or variety. Applying insecticides early in the season is expensive, may destroy the beneficial complex and often does not improve yield or profit. "One can succeed by simply doing nothing" (Wilson et al., 2003). Additional work is needed to clarify economic thresholds of migrating adult Lygus spp. populations sweeping, for short periods of time, through cotton fields at different times during the growing season.
Materials and Methods
Plot Design
Cotton was grown with high and low applications of nitrogen and water in split-plot, randomized complete block designs. Treatments consisted of four, five and six irrigations in 2003 and five irrigations in 2004, 2005, and 2006. Nitrogen fertilizer treatments consisted of one preplant broadcast application of 45 lbs N/acre and one side-dress application at layby to bring up total nitrogen levels to 45 and 135 lbs N/acre in 2003 and 2004; 50, 80, 115, 142, and 234 lbs N/acre in 2005; and 45, 80 and 135 lbs N/acre in 2006. One 80-lb N/acre application in 2006 was applied as a precision side-dress liquid application and compared to conventional45-, 80- and 135-lb N/acre side-dress granular applications. Stress was measured and recorded with a GreenSeeker™ and insects collected with an RV collector. NDVI readings for the precision nitrogen applications were set between 0.6 and 0.8. ANOVAs were conducted to compare plant responses from nitrogen and soil moisture to yield, development, lint quality and insect densities.
Results From 2003–2006
Results of NDVI/Nitrogen Experiments
Two years' data show a high correlation between average NDVI readings, yield and nitrogen levels (R2 = 0.93 and 0.83 in years 2004 and 2005) in cotton (Carrillo et al., 2006a, b & c). Nitrogen needs may be modified based on previous crops, nitrogen source, application methods, irrigations, rainfall, soil type, organic matter, crop variety, etc. There were no significant micronaire (fiber quality) differences between fertilizer and irrigation treatments. All treatments expressed similar height/node ratios, but higher water and nitrogen plots needed Pentia™ applications to hold back growth. Defoliants were needed only on the higher nitrogen/water treatments (142 and 234 lbs N/acre). There were no yield differences between conventional and precision applications of nitrogen at 80 lbs N/acre in 2006.
Results of Insect Experiments
The eradication of primary insect pests (boll weevil and pink bollworm) makes sustainable biological control of secondary insect pests (aphids, whiteflies, bollworm and Lygus spp.) practical in New Mexico. Our research focused on Lygus spp. control in 2003, 2004, 2005 and 2006. Predator/Lygus spp. adult ratios (P/L) were calculated each year by adding the absolute densities of the major adult and nymph predator groups (Nabis spp., Geocoris spp., Collops spp., Chrysoperla spp., Hippodamia spp. and Orius spp./100 row-ft) divided by the major absolute densities of the economic prey (Lygus spp. adults/100 row-ft). Three applications of chlorpyrifos in 2003 were not enough to completely control Lygus spp. adults (Figure 4) and did not decrease the density of the beneficial complex (total predators/100 row-ft averaged 79 and the P/L was 4.2–4.8). Five applications of chlorpyrifos controlled the Lygus spp. adult populations and decreased the density of the predatory complex (total predators/100 row-ft averaged 22.6 and the P/L was 5.4 and 7.2). In 2005, there were no insecticide applications. Predaceous insect densities were high and Lygus spp. adult densities low (total predators/100-ft averaged 70 and the P/L was 12.1–30.5 depending on nitrogen application rates).
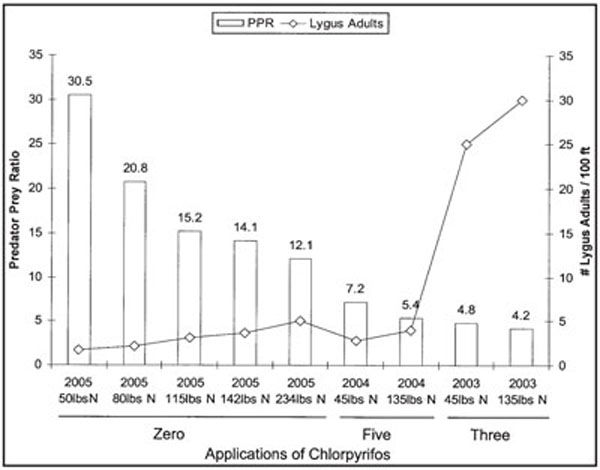
Figure 4. Predator/ Lygus spp. adult densities derived from 100-ft absolute vacuum samples from Acala 1517-99 cotton in 2003, 2004 and 2005. Three, five and zero chlorpyrifos insecticide applications were made in 2003, 2004 and 2005, respectively. Lygus spp. adult densities increased as nitrogen use rates increased but predator densities remained about the same in zero and low insecticide use years.
In 2006, very high migratory adult populations of Lygus spp. adults occurred (Figure 5). Densities spiked at nearly six times the normal rate, 80–140/100 row-ft, while total predators/100 row-ft averaged 68. P/R ratios were between 0.5 and 1.02. Even so, there were no yield differences attributable to Lygus spp.
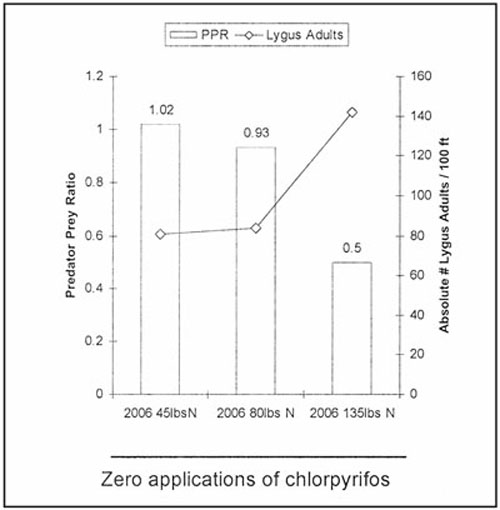
Figure 5. Predator/Lygus spp. adult density ratios derived from 100-ft absolute vacuum samples from DP555 cotton in 2006. The predator/Lygus spp. adult density ratios were doubled and the Lygus spp. adult densities nearly halved in the reduced nitrogen fertilizer applications (135–80 lb N/acre).
Increased nitrogen fertilizer rates increased the density of Lygus spp. adult populations but did not influence the density of the beneficial complex. Lygus spp. adult densities were decreased ~50% when nitrogen use rates were decreased from ~135 lbs to 80 lbs/acre (2005 and 2006). There were no significant micronaire (fiber quality) differences between fertilizer and irrigation treatments. All treatments expressed similar height/node ratios, but higher water and nitrogen plots needed Pentia™ applications to hold growth back. Defoliants were needed only on the higher nitrogen/water treatments (142 and 234 lbs N/acre). There were no yield differences between conventional and precision applications of nitrogen at 80 lbs N/acre in 2006.
Discussion
Nitrogen/Insect/Conservation Interactions
Lygus spp. densities are often lower in New Mexico than in neighboring states, perhaps because alfalfa fields interspersed with cotton fields act as alternative habitat for Lygus spp., a short growing season restricts biotic potential (Ellington et al., 1984b), and nitrogen application rates in New Mexico are on average half those in California and Arizona. From 1978–1993 California and Arizona rates were 113–178 lbs/acre and 106–140 lbs/acre, respectively, while rates in New Mexico during the interval 1978–1986 were 54–88 lbs N/acre, twice as high (Economic Research Service, 2006).
Average rainfall in July and August 2006 was 5.68 in. compared to a 10-year average of 2.40 in. from 1996–2005 (Mottt et al., 1992). Frequent rainfall may have encouraged the growth of alternate weed hosts for Lygus spp.; in addition, alfalfa hay cutting was delayed until the rain subsided. Many growers cut alfalfa hay late and at the same time in 2006, forcing high adult Lygus spp. populations into cotton. The control of adult Lygus spp. populations migrating from weeds and alternate cropping systems into cotton is a particularly difficult problem. Broad spectrum insecticide use further exacerbates this problem by decreasing the density of the beneficial complex, thus flaring in-field Lygus spp. populations. Solutions to this problem include strip cutting alfalfa hay, releasing Peristenus stygicus or P. digoneutis, and leaving border strips of alfalfa in hayfields to keep Lygus spp. adults there. Predator densities remained relatively stable in 2005 and 2006 when insecticides were not used.
Lygus spp. nymph densities were very low in 2003–2006 (1.17 average [range 0.5-14.5]/100 row-ft absolute), suggesting Lygus spp. populations in the Mesilla Valley, as in the San Joaquin Valley, are made up of mostly migrating adult populations (Rosenheim et al., 2006). Predators are not likely to control migrating adult Lygus spp., but they probably do help control eggs and nymphs of the next Lygus spp. generations. Predator and Lygus spp. adult densities resurged quickly after insecticide residues dissipated from three insecticide applications in 2003 but remained low after five insecticide applications in 2004. The very large between-year Lygus spp. density variations experienced during 2003–2006 suggest cotton fields should be sampled with precision equipment multiple times during the growing season to determine Lygus spp. densities, predator/prey ratios, adult/nymph ratios and their effect on yield and profit.
The densities of Lygus spp. and other harmful insects in New Mexico and other production areas can be reduced with proper cultural practices, by avoiding luxury consumption of nitrogen and water (Leigh, 1996; Altieri & Nicholls, 2003), and by providing habitat for beneficial insects (Garr, 2000). Other factors that influence Lygus spp. densities in specific fields include adjacent crops, timing of harvesting of adjacent crops, host weed populations, and crop variety. In most years and in most fields, Lygus spp. are not an economic problem in New Mexico cotton production.
Profit
Maximum yields do not necessarily equate to maximum profits, and future changes in technology at the farm level can be used to evaluate management decisions with this in mind. Many of the production costs for precision/conservation cotton production are similar to those for conventionally grown crops. Production cost differences occur primarily in soil fertility, pest management, irrigation, and plant growth regulator and defoliation expenses, which vary with different soil types, varieties, etc. An economic analysis1 was used to determine the best management strategies for cotton profit optimization in the Mesilla Valley. Five irrigations with 80 lbs of sidedress nitrogen per acre gave the highest profit (Carrillo et al., 2006c; Table 1). Insecticide, defoliation, excessive fertilizer and plant growth regulator expenses were deducted from the 80- and 50-lb N/acre treatments. Precision side-dress applications of nitrogen are expected to reduce nitrogen expenditures below those reported in Table 1.
Table 1. Irrigation number, pounds of sidedress nitrogen, yield and profit of Acala 1517-95 cotton at Las Cruces, New Mexico, 2005. Five irrigations with 80 lbs of nitrogen gave the optimum profit.
| Irrigations / lbs N | Yield (lbs/A) | Profit-Loss ($/A) |
| Five / 50 lbs | 1092 | +11.75 |
| Five / 80 lbs | 1341 | +117.00 |
| Five / 115 lbs | 1391 | +20.75 |
| Five / 142 lbs | 1492 | +70.02 |
| Five / 234 lbs | 1421 | +14.75 |
Conclusions
The precision/conservation farming methods used here have been designated the Fast Agricultural Response Management System (FARMS). The FARMS uses the following tools: NDVI (stress) readings, improved insect sampling, and computer insect counting tools. The use of these tools will result in lower use of nitrogen fertilizer, water, insecticide, plant hormone and defoliants, thus sustaining natural predator/prey ratios, reducing expenditures of time and money and improving profit margins. These tools can also be used to transition New Mexico growers to organic cotton production for increased profits. Essential elements of the FARMS can be applied to a wide variety of field and orchard cropping systems in the United States.
References
Adkisson, P. L. (1957). Influence of irrigation and fertilizer on populations of three species of mirids attacking cotton (Plant Protection Bulletin 6, pgs. 33-36). Rome: United Nations FAO.
Altieri, M. A. & Nicholls, C. J. (2003). Soil fertility management and insect pests: Harmonizing soil and plant health in agro ecosystems. Soil and Tillage Research, 72: 203-211.
Amin, A.A., Sawiers, Z., Carrillo, T. & Ellington, J. (1994). Comparison of dispersion and regression indices of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) on cotton in New Mexico (pp 1031-1032). Proceedings of the Beltwide Cotton Conference.
Butt, C.H., Walton, R.R., & Ivy, E.E. (1946). The cotton fleahopper in Oklahoma (Bulletin T-24). Stillwater: Oklahoma Agricultural Experiment Station.
Carriere, Y., Ellsworth, C., Dutilleul, P., Ellers-Kirk, C., Barkley, V., & Antilla, L. (2006). A GIS-based approach for areawide pest management: the scales of alfalfa, weeds, and cotton. Entomologia Experimentalis et Applicata. 118:203-210.
Carrillo T., Drake, J. & Ellington, J. (2006a). Nitrogen as a contributor to phytophagous insect density in Acala 1517-99 cotton, Gossypium hirsutum (L.). Manuscript submitted for publication.
Carrillo T., Drake, J. & Ellington, J. (2006b). Precision water and nitrogen application in Acala 1517-99 cotton, Gossypium hirsutum (L.). Manuscript submitted for publication.
Carrillo, T., Lillywhite, J., Drake, J., & Ellington, J. (2006c). A nitrogen budget calculator for Acala cotton. Manuscript submitted for publication.
Chapman, L.J. (2002). Cotton Scouting Handbook (ANR-409). Auburn University: Alabama Cooperative Extension Service.
Conway, G.R., & Pretty, J. (1991). Unwelcome Harvest: Agriculture and pollution. London: Earthscan.
Dicke, M., & Vet, L.E.M. (1999). Plant-carnivore interactions: Evolutionary and ecological consequences for plant, herbivore and carnivore. In H.Olff, V.K. Brown, R.H. Drent (Eds.), Herbivores: Between plants and predators. The 38th Symposium of the British Ecological Society (pp 483-520). Oxford: Blackwell Science.
Economic Research Service. (2006). U. S. Fertilizer Use and Price. Washington, DC: United States Department of Agriculture.
Ellington, J., Kiser, K., Cardenas, M., Duttle, J., & Lopez, Y. (1984b). The Insectavac: a high-clearance, high-volume arthropod vacuuming platform for agricultural ecosystems. Environmental Entomology, 13: 259-265.
Ellington, J., Carrillo, T. & Drake, J. (2003). Pecan integrated biological control. Southwest Entomology, 27: 45-46.
Ellington, J., Carrillo, T & Sutherland, C. (2001). Biological control options in New Mexico pecans. In Thirty-fifth Western Pecan Conference Proceedings, (pp 45-60).
Ellington, J.J. & El-Sokkari, A. (1986). A measure of the fecundity, ovipositional behavior, and mortality of the bollworm, Heliothis zea (Boddie) in the laboratory. Southwestern Entomology, 11: 177-193.
Ellington, J., Kiser, K., Ferguson Faubian, G. & Cardenas, M. (1984a). A comparison of sweepnet, absolute, and insectavac sampling methods in cotton ecosystems. Journal of Economic Entomology, 77: 599-605.
Ellington, J. & Southward, M. (1996). Quadrat sample precision and cost with a high-vacuum insect sampling machine in cotton ecosystems. Environmental Entomology, 28: 722-728.
Ellington, J., Southward, M. & Carrillo, T. (1997). Association among cotton arthropods. Environmental Entomology, 26: 1004-1008.
Ellington, J., Southward, M., Kiser, K. & Ferguson Faubian, G. (1988). Design-based sampling, a technique for estimating arthropod populations in cotton over large land masses. In L. McDonald, B. Manly, J. Lockwood and J. Logan (Eds.), Lecture Notes in Statistics: Vol. 55. Estimation and Analysis of Insect Populations (pp 445-457). Berlin: Springer-Verlag.
Fletcher, R.K. (1929). The unseen distribution of Heliothis obsolete on cotton in Texas. Journal of Economic Entomology, 22: 757-60.
Fletcher, R.K. (1941). The relation of moisture content of the cotton plant to oviposition by Heliothis armigera Hbn. and to survival of young larva. Journal of Economic Entomology, 24: 456-458.
Gaines, J.C. (1932). Migration and population studies of the cotton bollworm moth (Heliothis obsolete Fab.). Journal of Economic Entomology, 25: 769-72.
Gaines, J.C. (1933). Factors influencing the activities of the cotton bollworm. Journal of Economic Entomology, 26: 957-62.
Garr, G.M., Wrattten, S.D. & Barbosa, P. (2000). Success in conventional biological control of arthropods. In G. Garr and S. Wratten. Biological Control: Measures of success (pp 104-132). Klewer Academic Pub.
Gutierrez, A.P., Wang, Y., & Regev, U. (1979). An optimizing model for Lygus hesperus (Heteroptera: Miridae) damage in cotton: the economic threshold revisited. Canadian Entomologist. 111: 41-54.
Herrera, E. (2001). Nitrogen movement in the soil-pecan tree system (Guide 651). Las Cruces: Cooperative Extension Service, New Mexico State University.
International Fertilizer Industry Association. (2000). Fertilizer Consumption Report. World and Regional Overview and County Reports. Paris: Author. Available at: https://www.fertilizer.org.
Isley, D. (1946). The cotton aphid (Bulletin 462). Arkansas Agricultural Experiment Station, University of Arkansas, Fayetteville.
Kerby, T.A., Keeley, M. & Hohnson, S. (1987). Growth and development of Acala cotton (Bulletin 1921). Division of Agriculture and Natural Resources, University of California, Oakland.
Layton, M.B. (2000). Biology and damage of tarnished plant bug, Lygus lineolaris, in cotton. Southwestern Entomologist, Supplement 23, pp 7-20.
Leigh, T. (1996). Insect management. In Cotton Production Manual (Pub. 3352, p 417). Division of Agriculture and Natural Resources, University of California, Oakland.
Letourneau, D.K. (1988). Soil management for pest control: A critical appraisal of the concepts. In Global Perspectives on Agroecology and Sustainable Agricultural Systems (pp 581-587). Sixth International Scientific Conference of IFOAM, Santa Cruz, CA.
Loya, J. (2002). Preliminary studies on the integrated control of the pink bollworm in cotton. Unpublished doctoral dissertation, New Mexico State University, Las Cruces.
McGarr, R.L. (1942). Relation of fertilizers to the development of the cotton aphid. Journal of Economic Entomology, 35: 482-3.
McGregor, E. A. (1927). Lygus elisus: a pest of the cotton regions in Arizona and California (Tech. Bull. 4:1-15). Washington DC: USDA.
Mott, P., Sammis, T.S. & Jackson, R. (1992). Automatic weather data collection and processing. Computers and Electronics in Agriculture, 7: 337-345.
Murdock, W.W. (1969). Switching in general predators, experiments on predator specificity and stability of prey populations. Ecological Monographs, 39: 334-335.
Owen, W. L., Jr. (1962). Interrelations and control of insects attacking legumes and cotton (Misc. Pub. 570: 1-7). College Station: Texas Agricultural Experiment Station.
Ozkock, H.A. (1997). Effectiveness of unconventional and conventional insecticides on the density of the beneficial insect complex in cotton. Unpublished master's thesis, New Mexico State University, Las Cruces.
Pierce, J.B., Monk, P.E. & O'Leary, P.O. (In Press). Yield response and compensation from simulated bollworm injury in New Mexico. In Proceedings of the Beltwide Cotton Conferences. Memphis, TN: National Cotton Council.
Raun, W.R., Solie, J.B., Johnson, G.V., Stone, M.L., Whitney, R.W., Lees, H.L., Sembiring, H. & Phillips, S.B. (1998). Microvariability in soil test, plant nutrient and yield parameters in Bermuda grass. Soil Science Society of America Journal, 62: 683-690.
Raun, W.R., Solie, J.B., Johnson, G.V., Stone, M.L., Mullen, R.W., Freeman, K.W., et al., (2002). Improving nitrogen use efficiency in cereal grain production with optical sensing and variable rate application. Agronomy Journal, 94: 815-820.
Rigway, R.L. & Gyrisco, G.G. (1960). Effect of temperature on the rate of development of Lygus lineoloaris (Hemiptera: Miridae). Annals of the Entomology Society of America. 53:691-694.
Rosenheim, J.A. (2006). Estimating the impact of Lygus hesperus on cotton: The insect, plant and human observes as sources of variability. Journal of Environmental Entomology, 35: 1141-1153.
Schoonhoven, L.M., Jermy, T. & van Loon, J.J.A. (1998). Insect Plant Biology: From physiology to evolution. London: Chapman and Hall.
Scriber, J.M. (1984). Plant-herbivore relationships: Host plant acceptability (pp 159-202). In W. Bell and R. Carde (Eds.), The Chemical Ecology of Insects. London: Chapman & Hall.
Simone, C.B. (2005). Cancer and Nutrition. A Ten-Point Plan to Reduce Your Risk of Getting Cancer. New York: Avery Pub. Group.
Smil, Vaclav. (1997). Global population and the nitrogen cycle. Scientific American, 76-81.
Smith, G. L. (1942). California cotton insects (Bull. 660:1-50). Davis: California Agricultural Experiment Station.
Sevacherian, V. & Stern, V. M. (1973). Host plant preferences of lygus bugs in alfalfa interplanted cotton fields. Environmental Entomology. 3:761-766.
Sevacherian, V. & Stern, V.M. (1975). Movements of lygus bugs between alfalfa and cotton. Environmental Entomology. 4:163–165.
Soldaat L.L. & Vrieling, K. (1992). The influence of nutritional and genetic factors on larval performance of the cinnabar moth Tyria jacobaeae. Entomology Experimental Applications, 62: 29-36.
Stern, V. M., Mueller, A., Sevacherian, V. & Way, M. (1969). Lygus bug control in cotton through alfalfa interplanting. California Agriculture. 23: 8-10.
Stern, V. M., van den Bosch, R. & Leigh, T. F. (1964). Strip cutting alfalfa for lygus bug control. California Agriculture. 18: 4-6.
Stern, V.M., van den Bosch, R., Leigh, T. F., McCutcheon, O.D., Sallee, W.R., Houston, C.E. & Garber, M.J. (1967). Lygus control by strip cutting alfalfa (AXT-241. 13 pp.). University of California Agricultural Extension Service.
Stewart, S.D. & Gaylor, M.J. (1991). Age, sex, and reproductive status of the tarnished plant bug (Heteroptera: Miridae) colonizing mustard. Environmental Entomology. 20: 1387-1392.
Stewart, S.D. & Gaylor, M.J. (1994). Effects of age, sex, and reproductive status on flight by the tarnished plant bug (Heteroptera: Miridae). Environmental Entomology. 23:80-84.
Stovig, V. (2004). Bread and peace. Voices. Jan-Feb, 35-42.
Swezey S.L., Goldman, P., Bryer, J. & Nieto, D. (2004). Comparison between organic, conventional, and IPM cotton in the northern San Joaquin Valley, California. IPM in Organic Systems. XXII International Congress of Entomology. Brisbane, Australia.
Thomas, F.L. & Dunnam, E. W. (1931). Factors influencing infestation in cotton by Heliothis obsolete Fab. Journal of Econonomic Entomology, 24: 815-21.
USDA Economic Research Service. (2006). U.S. fertilizer use and mice. Washington, DC: USDA.
U.S. Environmental Protection Agency. (2006). About pesticides: 2000-2001 pesticide market estimates. Washington, DC: U.S. EPA.
Van Loon, J.J.A. & Dicke, M. (2001). Sensory ecology of arthropods utilizing plant infochemicals. In F.G. Barth & A. Schmid (Eds.), Ecology of sensing (pp. 253-270). Berlin: Springer Verlag.
Warden, L.B., House, W., Jackson, L.E. & Tangie, K.J. (1992). Modeling the fate of nitrogen in the root zone: management and research applications. Proceedings 1992 California Plant and Soil Conference: Decision making in an uncontrolled environment. California Chapter of the American Society of Agronomy.
White, T. (1993). The Inadequate Environment. Berlin: Springer-Verlag.
Wilson, L.J., Sadras, V.O., Heimoana, S.C. & Gibb, D. (2003). How to succeed by doing nothing. Crop Science, 43: 2125-2134.
1"Nitrogen use in cotton budget calculator". For more information contact J. Lillywhite at lillywhi@nmsu.edu.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu.
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
Printed and electronically distributed October 2007, Las Cruces, NM.


