Circular 609
D.B. Richman, J. Drake, T. Carrillo, S. Liesner, C.S. Bundy, J.J. Ellington
College of Agriculture, Consumer and Environmental Sciences New Mexico State University (Print Friendly PDF)
Authors: Respectively, Science Specialist, Adjunct Assistant Professor, Senior Research Specialist, Assistant Professor, Professor, all of Department of Entomology, Plant Pathology and Weed Science, New Mexico State University.
Abstract
A color key to spiders found in alfalfa and cotton in New Mexico is presented. Color digital photographs are placed at every couplet to aid in identifying specific morphological features. It is hoped that keys similar to the present one will allow non-specialists in the future to evaluate the beneficial complexes in their systems. Keys of this type should allow the lay person to make reasonably accurate identifications.
Introduction
Spiders have been collected and identified in the alfalfa and cotton fields of New Mexico, over the last 20 years, from the Mesilla Valley, Doña Ana County. Richman et al. (1990) outlined the fauna of alfalfa and compared it with faunas in New York, Virginia, Kentucky and California (Wheeler 1973, Howell & Pienkowski 1971, Culin and Yeargan 1983 a-c, Yeargan and Dondale 1974). Breene et al. (1993) published a review of spiders in Texas cotton fields. This publication included a picture key, utilizing published illustrations from various sources. There have been no publications on cotton spiders of New Mexico, but there was a preliminary presentation of the current study including the Doña Ana County fauna at an informal symposium prior to a national meeting of the Entomological Society of America (New Orleans, La., Dec. 2, 1990).
Whitcomb et al. (1963), Riechert and Lockley (1984), and Young and Edwards (1990) have all indicated that spiders are important predators in most, if not all, agricultural systems. Mansour (1987) indicated that spiders can control such pests in cotton as the Egyptian leaf worm. A more recent study by Riechert and Bishop (1990) in vegetable plots provides the best evidence that spiders can limit pest populations. Most recently, Wise (1993) summarized the literature on the impact of spiders on insect populations and called for more studies because some research seemed to be contradictory. Some problems in determining the role of spiders in agroecosystems in current and past research include documenting functional spider/prey responses, and the broad range of spider predatory habits and possible competition with and/or predation on other biological control agents. The general consensus seems to be that spiders, especially certain guilds of spiders, are important in limiting populations of certain pests, but that other guilds or species may actually be detrimental to other beneficials or neutral to pest species. This result should not be especially surprising because the system is basically indifferent to human activities. That the community of natural enemies functions as well as it does to limit pest most of the time is remarkable.
The majority of spiders collected in alfalfa and cotton in the Mesilla Valley were Pardosa sternalis (Thorell), Misumenops celer (Hentz), Misumenops coloradensis Gertsch, Grammonota cf. pictilis O.P. Cambridge and Tetragnatha laboriosa Hentz. These made up 95% of 3,473 spiders collected in alfalfa fields (Richman et al. 1990). The species of Misumenops and Misumenoides can probably be considered neutral or perhaps even harmful as they habitually wait to ambush prey on flower heads. They would thus be likely to capture many pollinators and parasitic Hymenoptera. The rest, especially the lycosid and linyphiid spiders (Pardosa, Erigone and Grammonota, among others), are probably beneficial. Pardosa sternalis is a hunting spider that actively searches for prey and may find more of the abundant pest species because of this trait. Grammonota and Erigone may build small sheet webs and may capture many tiny insects, especially aphids and leafhoppers, but little is known about their role in agroecosystems. Tetragnatha is an orb-weaver that can actually have an impact on pest insects, especially on aphid and mosquito populations (Provencher and Coderre 1987, Dabrowska-Prot and Luczak 1968). Thus three of the five most abundant spiders in alfalfa and cotton are probably beneficial in most (but probably not all) cases.
The following key was developed to allow the researcher, student and the farmer or agricultural agent to identify the spiders known or suspected to occur in alfalfa and cotton agroecosystems in the Mesilla Valley without recourse to the technical literature. It is hoped that with familiarity, the beneficial species will be recognized and that their roles in biological control will be evaluated to provide a more solid foundation for management decisions. Spiders, however, do not exist in a vacuum. All of the mortality factors, including spiders, insect predators, parasitoids, diseases, and weather conditions, need to be evaluated and placed in the context of treatment expense and crop value before control decisions are made. Even so, the “footsteps of the farmer” are the ultimate necessity in any agricultural situation.
How to Use the Key
The following key has been developed to facilitate the identification of common spiders in the alfalfa and cotton agroecosystems. This key should work well in all cotton and alfalfa growing areas, in the southern parts of New Mexico. We have tried to reduce terminology and to define terms when it is necessary. We are also including a speed key, which should help break down the possibilities before starting with the color key. Photographs of specimens should aid greatly in understanding the key.
Speed Key
| If the spider has enlarged chelicerae (mouthparts) and six eyes arranged in a partial circle (sowbug-eating spiders) see figs. 7-9 | Dysderidae |
| If the spider has enlarged anterior median eyes (jumping spiders) see fig. 17 | Salticidae |
| If the spider has enlarged posterior median eyes (wolf spiders) see fig. 51 | Lycosidae |
| If the spider was found on an orb (wheel-shaped) web (orb-weavers) see figs. 3-4, 46, 55-56, 62, 67, 72-73 | Tetragnathidae, Araneidae or Uloboridae |
| If the spider was found in a space (tangled) or tiny sheet web see figs. 5-6, 57-58, 63-66, 68-71 | Dictynidae, Theridiidae or Linyphiidae |
| If the spider has normal legs, eight eyes in two eye rows, and no prey capture web (may be found in silken, resting retreats or with egg sac) (sac and ground spiders) see figs. 26-27, 40-43 | Anyphaenidae, Miturgidae, Corinnidae or Gnaphosidae |
| If the spider has laterigrade legs (first three pairs flattened and facing forward) and no web (crab spiders) see figs. 32-33, 35-39 | Thomisidae or Philodromidae |
| If the spider has two rows of spines of different lengths on the front legs and feeds on other spiders (pirate spiders) see figs. 12-13 | Mimetidae |
| If the spider has six of its eight eyes arranged in a circlet (lynx spiders) see figs. 53-54 | Oxyopidae |
A Color Key to the Common Spiders Found in Alfalfa and Cotton in New Mexico
DYSDERIDAE or SOWBUG-EATING SPIDERS (NOTE: This European introduction is usually found with sowbugs and may have a mildly venomous bite.)NOTE: Also Hogna carolinensis Walckenaer and other genera and species, mostly larger in size than Pardosa.(NOTE: This spider has a powerful neurotoxin venom and should not be handled.) Fig. 65 shows a juvenile female.)(NOTE: None of these are these are considered dangerous.)| 1a. Spiders with a cribellum (sieve plate) (fig. 1) in front of spinnerets on venter; females and most males with calamistrum (comb) (fig. 2) on fourth metatarsus | 2 |
| 1b. Spiders without cribellum or calamistrum | 3 |
|
2a. Orb weavers (fig. 3 and fig. 4) |
Uloborus sp. (ULOBORIDAE or ORB-WEAVING CRIBELLATE SPIDERS) |
|
2b. Builders of tangle webs on plant terminals (figs. 5-6) |
Dictyna spp. (DICTYNIDAE or TINY SPACE-WEB WEAVERS) |
|
3a. Spiders with six eyes arranged in a partial circle (fig. 7); chelicerae (mouthparts) enlarged and with fangs arranged like ice tongs (figs. 8-9) |
Dysdera crocata C.L. Koch |
|
3b. Spiders with eight eyes, arranged usually in two rows (fig. 10); chelicerae “normal”, not ice-tong-like (fig. 11) |
4 |
|
4a. Spiders with two rows of spines of different lengths on tibia and metatarsus I and II (figs. 12-13) |
Mimetus sp. (MIMETIDAE or PIRATE SPIDERS) |
|
4b. Spiders without double row of spines of different lengths on tibia and metatarsus I and II (fig. 14) |
5 |
|
5a. Spiders with two claws on tips of tarsi sometimes obscured by claw tufts (fig. 15) |
6 |
|
5b. Spider with three claws (third claw difficult to see, but never with claw tufts (fig. 16) |
16 |
|
6a. Anterior median eyes enlarged; spiders often “notice” things around them (fig. 17) |
7 (SALTICIDAE or JUMPING SPIDERS) |
|
6b. Anterior median eyes the same size or smaller than other eyes (fig. 18) |
11 |
|
7a. Third legs longer than the others (fig. 19) |
Habronatus klauseri (Peckham & Peckham) |
|
7b. Third leg not longer than others (fig. 20) |
8 |
|
8a. Covered by iridescent green, pink, coppery, purple or golden scales; beetle-like (fig. 21) |
Sassacus papenhoei Peckham & Peckham |
|
8b. Not covered with iridescent scales or not beetle-like |
9 |
|
9a. With iridescent golden scales but elongated, not beetle-like (fig. 22) |
Sassacus vitis (Cockerell) |
|
9b. Without iridescent scales, may have iridescent mouthparts |
10 |
|
10a. Large spiders (8mm+) as adults; hairy and with iridescent green, blue or pink chelicerae (mouthparts) (fig. 23). Several species, including P. audax (Hentz), a common black and white species, often with an orange, white or red blotch or triangular spot and two white dash marks on the dorsum of the abdomen and green chelicerae (figs. 23-24). |
Phidippus spp. |
|
10b. Smaller spiders (3-4mm) as adults; yellowish females with black spots on abdomen or unmarked; less hairy males than Phidippus sp.,with yellowish or white striped chelicerae (fig. 25) |
Metaphidippus chera (Chamberlin) |
|
11a. Tracheal spiracle near middle of abdomen or epigastric furrow (fig. 26); yellowish or cream-colored spiders with small dark dots or bands on abdomen (fig. 27) |
Hibana incursa (Chamberlin) (ANYPHAENIDAE) |
|
11b. Tracheal spiracle near spinnerets (fig. 28) |
12 |
|
12a. Legs laterigrade, first three pairs flattened and facing forward (fig. 29) |
13 |
|
12b. Legs prograde (normal) not flattened, only first two pairs are facing forward (fig. 30) |
15 |
|
13a. Claw tufts present (fig. 31); usually with leaf shaped “heart” mark on abdomen (figs. 32-33) |
Ebo sp. Thanatus or Philodromus sp. (PHILODROMODAE) |
|
13b. Claw tufts absent (fig. 34); leaf shaped “heart” mark lacking (fig. 35) |
14 (THOMISIDAE) |
|
14a. Yellow, white or orange, flower dwelling species (figs. 36-38) [two species of Misumenops (fig. 36), which only can be separated by anatomical structures of the adults- M. coloradensis Gertsch and M. celer (Hentz) and one species of Misumenoides which can be separated from the Misumenops by the keel or ridge on the face (fig. 37) and the less obvious body hairs (fig. 38) |
Misumenops spp. & Misumenoides formosipes (Walckenaer) |
|
14b. Brown or dark-colored, bark or ground dwelling species (fig. 39) |
Xysticus spp. |
|
15a. Anterior spinnerets conical and touching at base (fig. 40) example of Miturgidae, Cheiracanthium inclusum (Hentz) yellow without markings, chelicerae black (fig. 41), example of Corinnidae,Trachelas mexicanus Banks (fig. 42) (Note: This species and the related Meriola decepta (Banks) resemble Dysdera crocata in color, but are smaller, have eight eyes arranged in two rows and have much smaller chelicerae.) |
(MITURGIDAE & CORINNIDAE or SAC SPIDERS) |
|
15b. Anterior spinnerets cylindrical and not touching at base or sometimes with touching spinnerets (fig. 43). (All gnaphosids lacking this trait are very small apparent ant mimics in the genus Micaria.) |
(GNAPHOSIDAE or GROUND SPIDERS – many genera and species) |
|
16a. Weavers of funnel-webs (figs. 44-45) or non-web weaving, running or ambushing spiders |
17 |
|
16b. Orb-web weavers (fig. 46) or tangle-web weavers (fig. 47) |
19 |
|
17a. Tarsal trichobothria (fine sensory hairs) in a single row (fig. 48); eyes in two rows, never in circle or in three rows (fig. 49) |
Agelenopsis spp. and Hololena hola (Chamberlin) (AGELENIDAE or FUNNELWEB-WEAVERS) |
|
17b. Tarsal trichobothria irregular, in two dorsal rows (fig. 50); eyes either appear to be in three rows (fig. 51) or in circle (fig. 53) |
18 |
|
18a. Posterior eyes enlarged, especially posterior median eyes; front eyes small and in single row (fig. 52) |
Pardosa sternalis (Thorell) (LYCOSIDAE or WOLF SPIDERS) |
|
18b. Eyes approximately the same size and arranged in a circle or hexagon plus two (figs. 53-54) |
Oxyopes salticus (Hentz) (OXYOPIDAE) |
|
19a. Chelicerae elongated and spiny (fig. 55), body elongated and often silvery (fig. 56); orb-web weavers |
Tetragnatha laboriosa Hentz (TETRAGNATHIDAE) |
|
19b. Chelicerae not elongate and spiny (fig. 57), body usually not elongated (fig. 58); tangle-web and orb-web weavers |
20 |
|
20a. Tarsus IV with comb of serrated hairs (fig. 59); tangle-web weavers |
21 (THERIDIIDAE) |
|
20b. Tarsus IV without comb of serrated hairs (fig. 60); tangle-web and orb-web weavers (figs. 61-62) |
22 |
|
21a. Black to white or spotted spiders with a distinct red to orange hourglass marking on the underside (figs. 63-65) |
Latrodectus hesperus Chamberlin & Ivie (WESTERN BLACK WIDOW) |
|
21b. Usually brown, often mottled (fig. 66) |
other Theridiidae e.g. Steatoda sp. (Comb-footed spiders) |
|
22a. Face (Clypeus) narrow vertically (fig. 67); orb-web weavers |
24 ARANEIDAE |
|
22b. Face wide vertically (fig. 68); tangle-web weavers |
23 LINYPHIIDAE |
|
23a. With spines around edge of carapace (fig. 69) and also see whole spider (fig. 70) |
Erigone sp. cf. aletris Crosby and Bishop |
|
23b. Without spines around edge of carapace (fig. 71) (and other species and genera). |
Grammonota sp. cf. pictilis O. P.-Cambridge |
|
24a. With distinctive bar on underside (fig. 72) |
Metepeira sp. |
|
24b. With distinctive spots on underside (fig. 73) |
Neoscona oaxacensis (Keyserling) |
Literature Cited
Breene, R.G., D.A. Dean, M. Nyffler and G.B. Edwards. 1993. Biology, predation ecology, and significance of spiders in Texas cotton ecosystems with a key to the species. Bull, Texas A. &M. Exp. Stn. B-1711:1-115.
Culin, J.D., and K.V. Yeargan. 1983a. Spider fauna of alfalfa and soybean in Central Kentucky. Trans. Ky. Acad. Sci. 44:40-45.
Culin J.D. and K.V. Yeargan. 1983b. Comparative study of spider communities in alfalfa and soybean ecosystems: Foliage-dwelling spiders. Ann. Entomol. Soc. Amer. 76:825-831.
Culin J.D. and K.V. Yeargan 1983c. Comparative study of spider communities in alfalfa and soybean ecosystems: Ground-surface spiders. Ann. Enotmol. Soc. Amer. 76:832-838
Dabrowski-Prot, E., and J. Luczak. 1968. Studies on the incidence of mosquitoes in the food of Tetragnatha montana Simon and its food activity in the natural habitat. Ekologia Polska-Seria A 16:843-854.
Howell, J.O., and R.L. Pienkowski. 1971. Spider populations in alfalfa, with notes on spider prey and effect of harvest. J. Econ. Entomol. 64:163-168.
Mansour, F. 1987. Spiders in sprayed and unsprayed cotton fields in Israel, their interactions with cotton pests and their importance as predators of the Egyptian cotton leaf worm, Spodoptera littoralis. Phytoparasitica 15:31-41.
Provencher, L., and D. Coderre. 1987. Functional responses and switching of Tetragnatha laboriosa Hentz (Araneidae: Tetragnathidae) and Clubiona pikei Gertsch (Araneae: Clubionidae) for the aphids Rhopalosiphum maidis (Fitch) and Rhopalosiphum padi (L.) (Homoptera: Aphididae). Environ. Entomol. 16:1305-1309.
Richman, D.B., J.J. Ellington, K.R. Kiser and G. Ferguson-Faubion. 1990. A comparison of a New Mexico alfalfa spider fauna with eastern and California faunas. Southwest Entomol. 15:387-397.
Riechert, S.E., and L. Bishop. 1990. Prey control by an assemblage of generalist predators: spiders in garden test systems. Ecology 71:1441-1450.
Riechert, S.E., and T. Lockley. 1984. Spiders as biological control agents. Ann. Rev. Entomol. 29:299-320.
Wheeler, A.G. Jr. 1973. Studies in the arthropod fauna of alfalfa. V. Spiders (Araneida). Can. Entomol. 105:425-432.
Whitcomb, W.H. 1974. Natural populations of entomophagous arthropods and their effect on the agroecosystem, pp. 150-169. In, F.G. Maxwell and F.A. Harris. ed. Proceedings of the Summer Institute on Biological Control of Plant Insects and Diseases, Stoneville, Mississippi.
Whitcomb, W.H., H. Exline and R.C.Hunter. 1963. Spiders of the Arkansas cotton field. Ann. Entomol. Soc. Amer. 56:653-660
Wise, D.H. 1993. Spiders in ecological webs. Cambridge Univ. Press, New York, 328p.
Yeargan, K.V. and C.D. Dondale. 1974. The spider fauna of alfalfa fields in Northern California. Ann. Entomol. Amer. 67:681-682.
Young, O.P. and G.B. Edwards. 1990. Spiders in United States field crops and their potential effect on crop pests. J. Arachnol. 18:1-27.
Spider Figures (1-73)

Figure 1

Figure 2

Figure 3

Figure 4

Figure 5

Figure 6

Figure 7

Figure 8

Figure 9

Figure 10

Figure 11

Figure 12

Figure 13

Figure 14

Figure 15

Figure 16

Figure 17

Figure 18

Figure 19

Figure 20

Figure 21

Figure 22

Figure 23

Figure 24

Figure 25

Figure 26

Figure 27
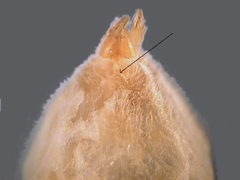
Figure 28

Figure 29

Figure 30

Figure 31

Figure 32

Figure 33

Figure 34

Figure 35

Figure 36

Figure 37

Figure 38

Figure 39
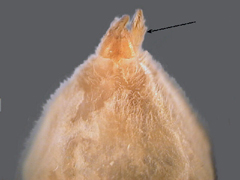
Figure 40

Figure 41

Figure 42
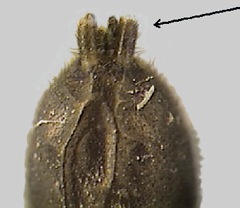
Figure 43

Figure 44

Figure 45

Figure 46

Figure 47

Figure 48

Figure 49

Figure 50

Figure 51

Figure 52

Figure 53

Figure 54

Figure 55

Figure 56

Figure 57

Figure 58

Figure 59

Figure 60

Figure 61

Figure 62

Figure 63

Figure 64

Figure 65

Figure 66

Figure 67

Figure 68

Figure 69

Figure 70

Figure 71

Figure 72

Figure 73
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu
Contents of publications may be freely reproduced for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
April 2006, Las Cruces, NM.