Guide B-235
John C. Wenzel
College of Agricultural, Consumer and Environmental Sciences, New Mexico State University
Author: Extension Veterinarian, Department of Extension Animal Sciences and Natural Resources, New Mexico State University. (Print Friendly PDF)
Introduction
Antimicrobial drugs are widely used in livestock production. They are used to prevent, control, and treat many types of microbial infections and exposures encountered by livestock. As our scientific knowledge increases regarding the unintended consequences of using antimicrobials, we must adjust our thinking and production practices to include decisions based on judicious use of antimicrobials and the best management practices associated with judicious use. Using antimicrobials incorrectly can contribute to increased antimicrobial resistance (AMR). This is a concern for both human and animal health, and is the reason for changing the way that the livestock industry will use antimicrobials in the future.

The Food and Drug Administration (FDA) has issued guidelines for using Medically Important Antimicrobial Drugs (MIAD), including Guidance for Industry (GFI) #152, #209, and #213 and the Veterinary Feed Directive (VFD). The principles in these documents for antimicrobial usage need to become the standard for using these products in all segments of the livestock industry. Antimicrobial resistance is a very complex problem with no single cause or solution. It will take a concerted effort by everyone involved in both human and livestock health to face this challenge and modify behaviors that may contribute to AMR.
Basic Definitions
- Antibiotic: A naturally occurring compound (drug) that fights infection caused by bacteria by either killing them (cidal drugs) or inhibiting their growth (static drugs).
- Antimicrobial: Does the same as antibiotics, but also includes synthetic compounds, including compounds that act on other microorganisms besides bacteria, such as antivirals, antifungals, and parasiticides. All antibiotics are antimicrobials, but not all antimicrobials are antibiotics.
- The New Mexico Veterinary Practice Act defines a “valid veterinarian-client-patient relationship” (VCPR) to exist when:
- Prescription drug (Rx): A drug or medication for use by, or on the order of, a licensed veterinarian. This requires veterinary oversight and a valid VCPR.
- Over-the-counter (OTC) drug: A drug or medication that does not require veterinary oversight and can be purchased and used by anyone. When using an OTC drug, you must follow the label directions exactly or you are in violation of the law unless extra label drug use guidelines are followed (see below). GFI #263 sets the deadline of June 2023 that all antibiotics will need veterinary oversight. OTC antibiotics will not be available after June 1, 2023.
- Labeled drug use (LDU): Any purchased drug or medication must be used EXACTLY as stated on the label. Legally, no deviation from labeled directions is allowed, including any variation in species, indication, dosage, route, frequency, timing, or withdrawal time, unless a valid VCPR is established and your veterinarian prescribes the variation (known as extra label drug use). This includes OTC and Rx medications.
- Extra label drug use (ELDU): To use a drug or medication in a way that deviates from labeled directions in any form or manner. To legally use a drug extra label, it must be prescribed by a licensed veterinarian under a valid VCPR, and carry additional label directions for use and a stated withdrawal time on that label.
Drug Labels
The label contains all the information necessary for the use of the particular drug, and you should be familiar with all of the label information prior to use. The livestock species, class, indications, dosage, administration, and withdrawal requirements must be followed exactly for legal use unless your veterinarian prescribes a variance or ELDU. If a particular species is not listed on the label, it may only be used if ELDU is permitted for that species.
The following is an example of some of the information from the label of the antimicrobial drug DRAXXIN that describes the species, class, indications, dosage and administration, warnings, and withdrawal requirements.
Beef and Non-lactating Dairy Cattle
BRD - DRAXXIN® Injectable Solution is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis; and for the control of respiratory disease in cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis.
IBK - DRAXXIN® Injectable Solution is indicated for the treatment of infectious bovine keratoconjunctivitis (IBK) associated with Moraxella bovis.
Foot Rot - DRAXXIN® Injectable Solution is indicated for the treatment of bovine foot rot (interdigital necrobacillosis) associated with Fusobacterium necrophorum and Porphyromonas levii.
Suckling Calves, Dairy Calves, and Veal Calves
BRD - DRAXXIN® Injectable Solution is indicated for the treatment of BRD associated with M. haemolytica, P. multocida, H. somni, and M. bovis.
Dosage and Administration
Cattle: Inject subcutanesously as a single dose in the neck at a dosage of 2.5 mg/kg (1.1 mL/100 lb) body weight (BW). Do not inject more than 10 mL per injection site.
Warnings
For use in animals only. Not for human use. Keep out of reach of children. Not for use in chickens or turkeys.
Residue Warnings
Cattle: Cattle intended for human consumption must not be slaughtered within 18 days from the last treatment. This drug is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
New Animal Drug Ranking
When a New Animal Drug Application is filed with the FDA, one of the considerations for the new drug is the ranking of importance for use in human medicine. This is explained in FDA Guidance for Industry #152. In Appendix A of GFI #152, the criteria used in ranking the importance of antimicrobial drugs, with regard to their relative importance in human medicine, is described in part as follows:
After careful consideration of many factors, the specific antimicrobial drugs or classes of antimicrobials are ranked as to whether they are critically important, highly important, or important to human medical therapy. Assignment of a ranking to a given antimicrobial or class of antimicrobials depends upon the degree to which multiple factors apply to the drug in question.
Critically Important drugs (and highest priority):
- Fluoroquinolones (all): Baytril, Advocin (formerly A180). ELDU prohibited.
- Macrolides: Tylan 40, Micotil, Draxxin, Zactran, Zuprevo. ELDU permitted with VCPR.
- Third-generation Cephalosporins: Naxcel, Excenel, Excede. ELDU prohibited in cattle and swine.
- Trimethoprim/Sulfa: Sulfa-trim, Uniprim. ELDU permitted with VCPR.
Highly Important drugs. Can be used ELDU with a valid VCPR where permitted:
- Penicillins: Pen BP, Procaine Pen G, Pen V
- Aminopenicillins: Amoxicillin, Ampicillin
- Aminoglycosides: Gentamicin (prohibited for use in food animals), Spectinomycin, Amikacin, Kanamycin, Tobramycin, Neomycin, Netilmicin
- Fourth-generation Cephalosporins: Cefepime
- Tetracyclines: Chlortet, Oxytet
Important drugs. Can be used ELDU with a valid VCPR where permitted:
- First- and Second-generation Cephalosporins
- Fourth-generation Cephalosporins: Cephamycins
Laboratory Testing of Antimicrobial Sensitivity
In any given infection, isolates of bacteria will vary in their response to the antimicrobial being tested. In the laboratory, one common method to determine a “sensitivity” pattern is based upon the susceptibility of the cultured bacteria to the effects of the antimicrobial disc placed on the culture media. This is based upon the minimum inhibitory concentration (MIC), which is the concentration of the drug in the media (tissue) that would reasonably be expected to inhibit growth of a large percentage of the target organism. This is determined by the size of the “zone of inhibition” (Figure 1), based on the diffusion of the antimicrobial through the culture media that is being used to grow the bacteria. The zone of inhibition is measured and results in the bacterial culture being classified into one of three classifications for each antimicrobial disc used in the sensitivity test:
- Sensitive: A clear zone of inhibition appears around the antibiotic disc, which shows an absence of growth of the target bacteria, indicating effectiveness of the antibiotic—at least in the laboratory setting.
- Intermediate: A definite zone of inhibition is not clear, indicating that some members of the bacterial culture were not susceptible to the antibiotic in the disc.
- Resistant: Little or no zone of inhibition present.
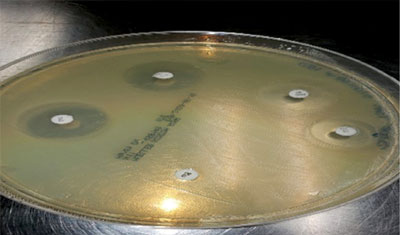
Figure 1. Zone of inhibition. The disc on the left shows a large zone of inhibition, while the bottom disc shows no inhibition.
Culture and sensitivity results from samples submitted to a diagnostic laboratory may not fully reflect the actual picture of the infection in the animal. Generally, mixed infections are the most prevalent, and some organisms are very difficult to isolate. The isolated bacteria may be the one that grew the best, not the one that was responsible for the disease in the animal. Laboratory results should be considered as additional information along with the clinical findings. The results of a culture and sensitivity test at a diagnostic laboratory can be a valuable tool used by your veterinarian in choosing the best targeted therapy in disease treatment.
FDA Guidance for Industry #209: Recommended Principles
- Use of medically important antimicrobial drugs in food-producing animals should be limited to those uses that are considered necessary for ensuring animal health.
- Use of medically important antimicrobial drugs in food-producing animals should be limited to those uses that include veterinary oversight or consultation.
- Feed efficiency and growth promotion are not judicious use, so those indications were removed from labels.
- Treatment is considered judicious.
- Control/prevention is judicious in certain instances.
General Principles for Antimicrobial Use
- Communicate and consult with your veterinarian—give them all the information and clinical signs possible. Try to determine what “system” is involved, such as the respiratory system versus the digestive system, and let your veterinarian know.
- When possible, the pathogen should be identified and antimicrobial susceptibility testing used to help determine the best antimicrobial to choose.
- Which antimicrobial to use should be based on what is the best choice for the suspected pathogen, with the economics of this choice being of secondary importance.
- As cow/calf producers, it is our responsibility to use all the technology available when developing preventive and herd healthcare options. Preventing disease through other management factors will help decrease the overall need for antimicrobials.
- Developing the immune system in the calves is the key to preventing bovine respiratory disease (BRD), the most costly and most treated bovine disease.
-
Good calving management and adequate colostrum intake will help decrease calf scours and other calfhood diseases. Colostral immunity is the first step in developing immunocompetent calves—take care of the cows!
-
Everyone involved in the cattle industry should take antimicrobial resistance seriously. Ag groups have adopted judicious use guidelines, and all segments of the industry should follow these guidelines.
Remember to follow Beef Quality Assurance (BQA) recommendations when administering any medications to food animals. All injections should be in front of the shoulder in the neck region, whether intramuscular or under the skin (Figure 2). Give no more that 10–15 cc in any one location, and separate injections by 5 inches or more.
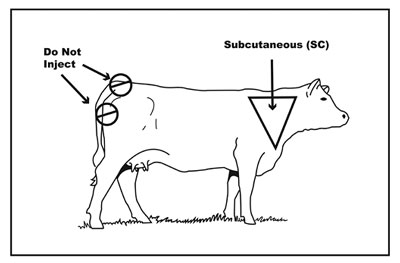
Figure 2. Proper injection site for cattle.
Preventive healthcare and judicious antimicrobial usage should be a priority of all livestock producers. It is our responsibility to the consumer to reduce antimicrobial usage by using all the technology available to prevent disease in livestock under our stewardship.
References
U.S. Food and Drug Administration. 2003, October 23. Guidance for industry #152: Evaluating the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern. Retrieved June 15, 2020, from https://www.fda.gov/media/69949/download
U.S. Food and Drug Administration. 2012, April 13. Guidance for industry #209: The judicious use of medically important antimicrobial drugs in food-producing animals. Retrieved June 15, 2020, from https://www.fda.gov/media/79140/download
Veterinary Practice Act, NMSA 1978, § 61-14-2.N. 1978.
For Further Reading
B-223: Calf Vaccination Guidelines
https://pubs.nmsu.edu/_b/B223/
B-224: Cow Herd Vaccination Guidelines
https://pubs.nmsu.edu/_b/B224/
B-226: Increasing the Effectiveness of Modified Live Vaccines
https://pubs.nmsu.edu/_b/B226/

John C. Wenzel is the Extension veterinarian in the Extension Animal Sciences and Natural Resources department at NMSU. He earned his B.S. from NMSU and his DVM from Kansas State University College of Veterinary Medicine. His work focuses on cow/calf medicine and preventative health programs for livestock producers in southwestern New Mexico.
Brand names appearing in publications are for product identification purposes only. No endorsement is intended, nor is criticism implied of similar products not mentioned. Persons using such products assume responsibility for their use in accordance with current label directions of the manufacturer.
To find more resources for your business, home, or family, visit the College of Agricultural, Consumer and Environmental Sciences on the World Wide Web at pubs.nmsu.edu.
Contents of publications may be freely reproduced, with an appropriate citation, for educational purposes. All other rights reserved. For permission to use publications for other purposes, contact pubs@nmsu.edu or the authors listed on the publication.
New Mexico State University is an equal opportunity/affirmative action employer and educator. NMSU and the U.S. Department of Agriculture cooperating.
December 2021 Las Cruces, NM


